As I work on my vindication article (it is about superantigens and how this brought a lot of answers to many of our questions and oddities regarding SARS-CoV-2… taking longer than expected), a rather fantastic paper was published.
I have a significant interest in minimizing, reversing and potentially enhancing systemic metabolism, with an interest in the effect of different metabolic states in immune cells and the immune system in the past few years. After dedicating a year and change to studying coronaviruses, in 2021 I suggested a ketogenic diet, but especially “exogenous ketones” (supplementing with ketones) was both protective against the infection and a resource to recover immune function and overall improve health.
The list of, often systemic, positive effects ketones have in our body is sizable, to say the least, it will “revert” T-Cell exhaustion, it can enhance CD8 (the cells that kill stuff and cancer), improve endothelial health, metabolic damage, improve glycemic control (among the most important aspect of our modern lives), improve inflammation but per the last article linked, it has significant, profound effects in the brain.
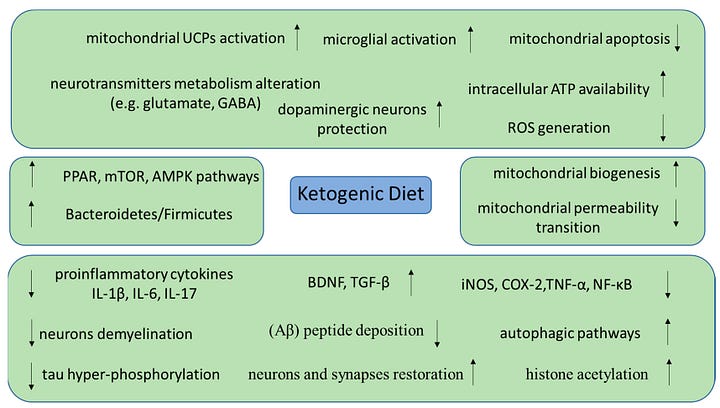
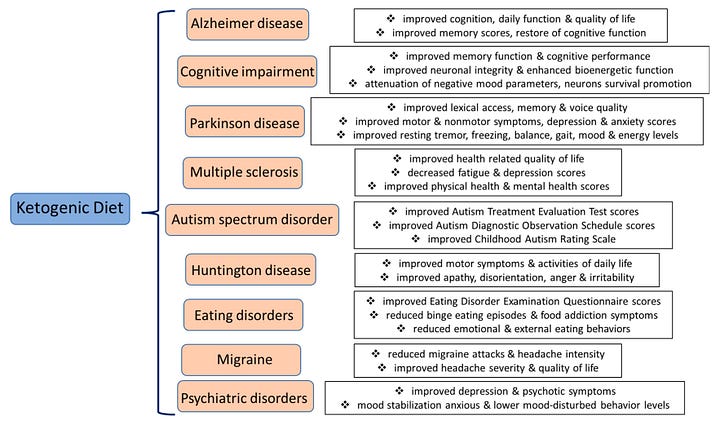
Now we have evidence for a novel effect, which is below my markdown map.
β-hydroxybutyrate is a metabolic regulator of proteostasis in the aged and Alzheimer disease brain
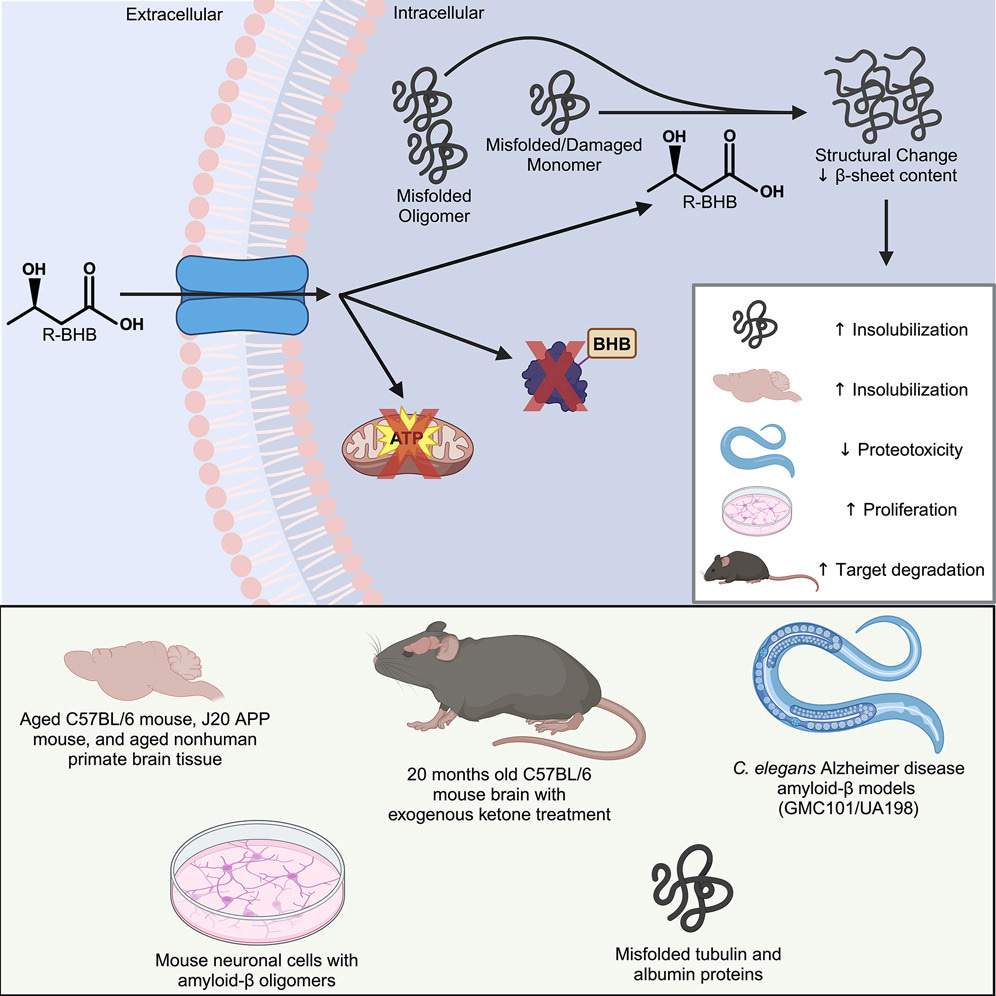
Ketone bodies, small molecules that provide lipid-derived energy to cells during fasting, have been linked to various mechanisms of brain aging and increased healthy longevity in mice, and other fasting metabolism mechanisms have been linked to proteostasis. These data fill an important puzzle piece in the literature on pathogenic protein clearance under varying metabolic states. Here, we provide a direct molecular mechanism for the regulation of misfolded proteins by ketone bodies and related metabolites. While many factors can affect protein solubility in vitro, we showed that this mechanism is robust and reproducibly not dependent on covalent protein modification, pH, or solute load. Importantly, we reproduced the ex vivo effect in vivo, using a ketone ester to indirectly deliver exogenous R-βHB to the mouse brain via hepatic metabolism to R-βHB and physiological transport of R-βHB into the brain without other exogenous biochemical alterations. R-βHB insolubilization targets that we identified ex vivo strongly overlap with targets found in vivo, supporting the similarity of mechanism between the ex vivo and in vivo systems. We validated the physiological relevance of the mechanism by showing rescue in cell-based and C. elegans models of amyloid-β proteotoxicity. Given that proteostatic mechanisms like autophagy are known to be activated by nutrient deprivation, it is unsurprising that evolutionary pressures would encourage the clearance of pathogenic proteins during ketosis to promote cellular health in organisms seeking additional substrate for ATP production. In this situation, ketone bodies are janitors of damaged proteins, chaperoning away molecular waste so organisms can operate at peak molecular fitness. This mechanism can be leveraged for therapeutic development in aging and NDDs, including via pharmacological approaches for which we provide proof of principle with BH-BD. Understanding the molecular mechanisms of metabolism is an essential aspect of the future of accessible therapeutic interventions in aging and NDDs.
Highlights
• βHB regulates protein solubility and is selective for a misfolding structural state
• βHB-induced insolubility is not dependent on covalent modification, pH, or solute load
• Protein targets of βHB include neurodegeneration-related proteins such as amyloid-β
• βHB effects are observable in vitro, ex vivo, and in vivo in nematode and mouse models
The overall findings in this paper are rather remarkable. The evidence so far shows that BHB can affect protein solubility by Post-Translation Modification, meaning the protein changes via chemical reactions or additions to it, but there the authors tested and found that BHB directly alters the protein surface, so its effect is direct interaction with the protein.
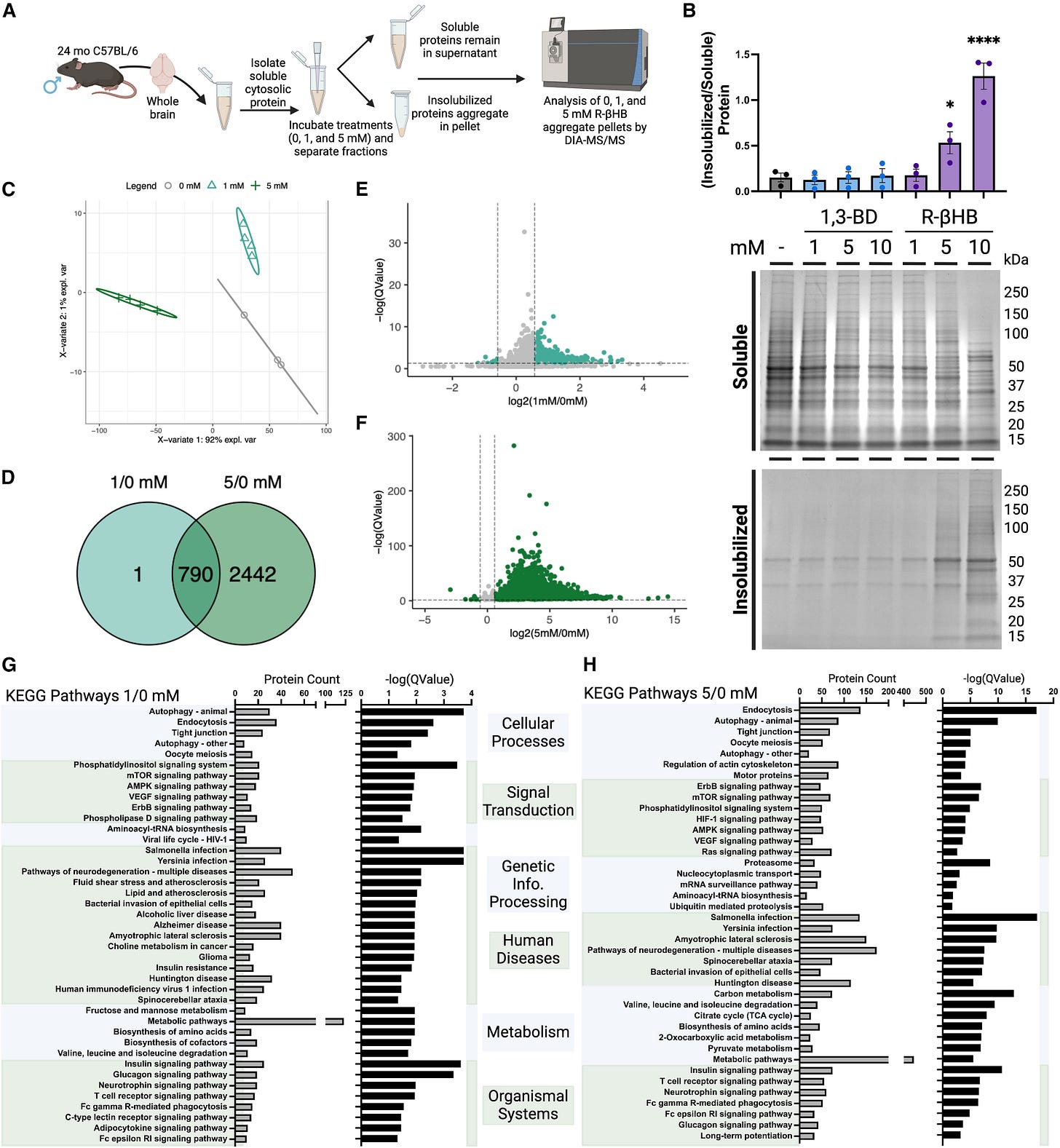
By using brain lysates (basically liquified brain) from aged mice the authors found that BHB targets multiple protein types, specifically high-molecular-weight ones associated with neurodegeneration. BHB prefers to attach itself to misfolded proteins.
Next they aimed to identify which proteins are targeted by BHB by using proteomics, which analyzes large-scale proteins, quantity, structure, and interactions, again using mouse brain lysate, and treating them with physiologically relevant concentrations (1–10 mM) simulating the average and peak activity.
The analyses revealed that BHB targeted protein involved in critical cellular functions such as vesicle transport, cellular catabolism, and especially important here neurodegeneration-related pathways. Proteins associated with Alzheimer’s, Huntington’s, and ALS were among them. Higher concentrations had a broader range of targeted proteins, meaning a dosage-dependent effect. Analyzing the genes involved they found proteins key to biological processes like protein degradation via autophagy and the ubiquitin-proteasome system.
The authors hypothesized the protective effects of BHB are structural in nature and sought to understand it. They used a Thioflavin T fluorescence test, widely used to monitor the content of beta-sheets content in proteins. Beta sheets are the twisted proteins that are the “structural basis” of amyloid proteins.
Brain lysates from aged mice were used again, and treated with BHB, and the results showed a significant reduction in Beta-sheet content, and the higher the BHB concentration, the lower the content, suggesting BHB either disrupts or directly prevents amyloid structure formation. This effect was consistent in other models, such as transgenic J20 mice (genetically modified mice to express a lot of amyloid beta), and even in primates.
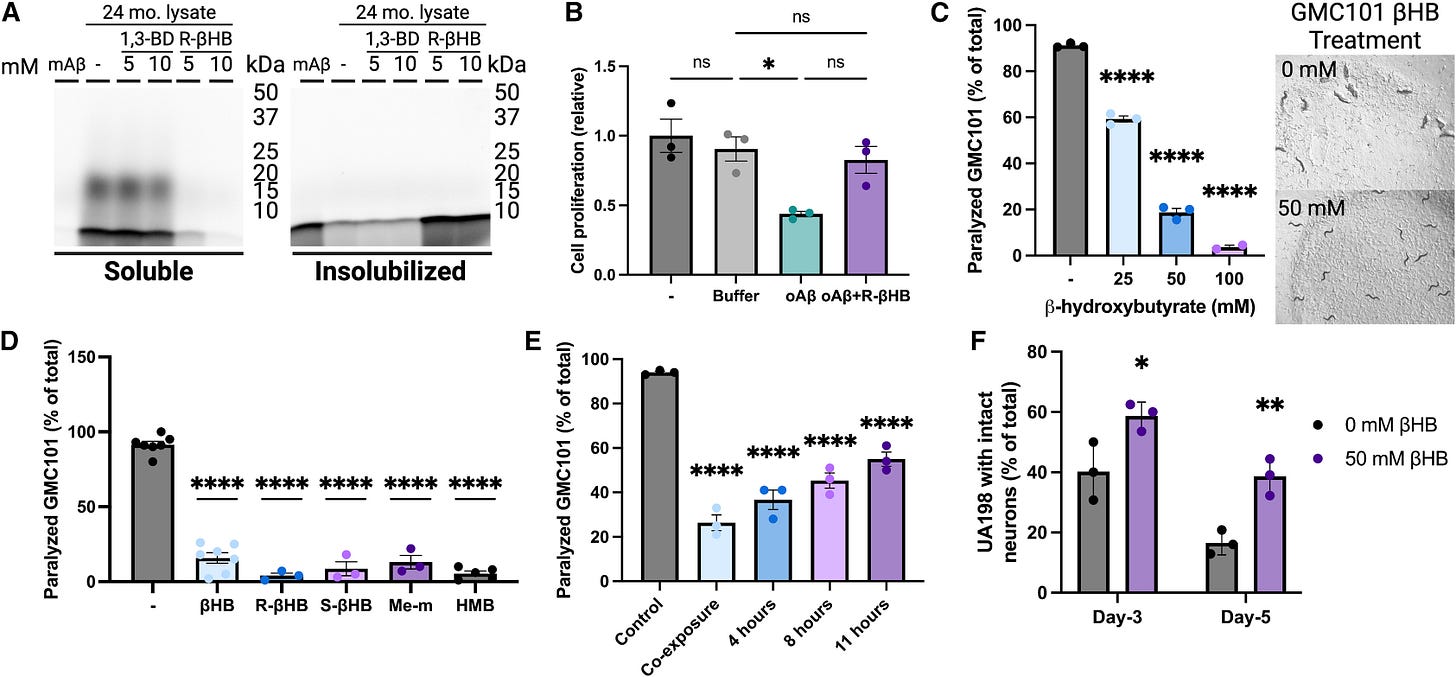
The structural effects of BHB translated into functional benefits, particularly against amyloid-beta (Aβ), the notorious misfolded protein associated with Alzheimer’s disease. BHB treatment inhibited Aβ aggregation and oligomeric toxicity, the latter being especially significant as small oligomers are among the most toxic forms of Aβ. Even the highly aggregation-prone and toxic Aβ1-42 form was affected, transitioning from soluble, toxic oligomers into a less soluble state. This structural shift made Aβ less toxic across various experimental setups. Cellular assays using mouse neuroblastoma cells (N2a) confirmed these findings, showing that BHB restored cell viability even in the presence of Aβ oligomers.
To see if these are replicated at an organism level, the authors used a well-known amyloid-beta model, C. Elegans, a roundworm. A significant decrease in the percentage of animals experienced proteotoxicity-induced paralysis was observed at all BHB concentrations with the highest concentration completely rescuing the worms.
When they used the UA198 (worm that expresses specifically human amyloid beta 1-42) BHB-treated animals retained significantly higher proportions of intact neurons, with day-5 BHB-treated animals having equivalent intact neurons as day-3 control animals. BHB treatment both preserves and increases the survival of neural cells. In summary, all models tested either back their initial proposition, or further elucidated the mechanism by which BHB helps clear the brain of misfolded proteins.
It is interesting to note as a closing remark to their paper that BHB quite literally only targets unstable proteins, either unfolded, misfolded, or damaged proteins, meaning there is a relative specificity in BHB helping the body change, and clear bad proteins.
Why is this important ? Because it demonstrates that not only the ketogenic diet, but also supplementation with ketones has a significant impact on our time’s therapeutic effect, and it is one of the reasons I have recommended the supplementation with exogenous ketones allied with a lower carb (and either high fat or high protein diet) as means to mitigate, rescue, recover and improve any potential negative impact SARS-CoV-2 and its plaguesque Spike Protein could have both short and long term.
I will publish my Superantigen article before my birthday (December 9).
I am grateful for your support, it enables me to do my interdisciplinary and rather unorthodox research.











This is exciting to read. I changed my diet to high fat and low carb and actually lost weight and have more energy. Weight has never been a problem for me but I like to stay in shape and noticed the difference when I changed my diet. I want to get the practical side of supplementing with BHB before adding it so I look forward to your next pre birthday publication.
I had Delta C-19 in 2021 and have dealt with histamine intolerance since. Taking tributyrin, histaminX and eating a low histamine diet has helped but looking for further improvement.
Thank you for your hard work, it’s appreciated.
The evidence for fasting and ketosis just grows and grows. You said it some time ago, fix your diet and exercise first, the multitude of benefits just gets more and more compelling. I would even suggest a 48-72 hour fast if you have Covid or any other viral infection would probably be one of the best actions you could take.