The Long Shadow of the Kynurenine Pathway
Thrombosis, Neuroinflammation, Unforeseen Consequences
Sometimes, the stars align. There is an endless stream of metabolic, immunologic, and cellular pathways for one to choose from, delve into, and dedicate weeks, months, and years to studying, and learning. After all, the human physiology complexity in unparalleled, in so far that eve the most cutting-edge AI models barely scratch the surface.
From my experience, the Kynurenine Pathway stands out as one of the most curiosity-inducing – a true "fun to learn and solve" puzzle – and profoundly impactful biochemical cascades in the body. It touches upon our most vital organs, and each step, each byproduct within it, seems to possess a paradoxical, double-edged nature. If HMGB1, "The One Protein to Rule Them All", is biology's double-edged sword embodied in a single protein, then the Kynurenine Pathway is the poster child for a double-edged pathway itself.
A quote from the article above.
Whenever you read about the Kynurenine pathway, you will inevitably see or read about the Aryl Hydrocarbon Receptor (AhR), which the Kynurenine Pathway directly modulates, and so does a myriad of other things. Clots activate the “immune cells” in your brain via the AhR. Kynurenine is a player in thrombosis, in the kidney, and heart.
I’ve long stated the Kynurenine Pathway and its byproduct play a quasi-central role in the complex cascade that leads to clotting, especially microclotting. Alas scientific validation arrives away late to our party.
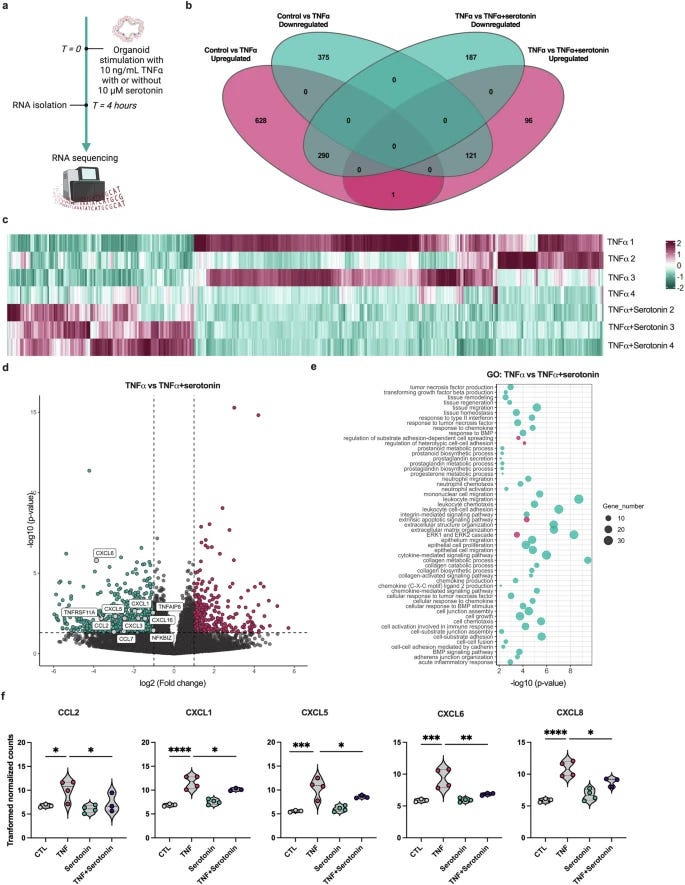
Tryptophan metabolism reprogramming contributes to the prothrombotic milieu in mice and humans infected with SARS-CoV-2
To preface this, a touch of biochemistry is needed, perhaps welcomedSeveral enzymes initiate the breakdown of tryptophan – TDO (Tryptophan 2,3-dioxygenase), IDO1, and IDO2. These "rate-limiting enzymes" act as gatekeepers, controlling the speed at which tryptophan is channeled into specific metabolic branches. TDO predominantly resides in the liver, while IDO exhibits a more ubiquitous presence, found across diverse cell types and tissues.
Given different teams focus on different organs and different testing methods, the authors decided to measure the expression of both TDO and IDO in humanized mice, a common practice. TDO in the liver increased 3x at 2 dpi (Days post-infection), and 5x at 4dpi, reverting back at 7 dpi. Measuring IDO in the kidneys and lung they found an increase of 3x at dpi in the renal tubules, remaining elevated at 7dpi.
They also found a downregulation of both KMO (Kynurenine-3-Monooxygenase) and KYNU (L-Kynureninase) at 4 and 7dpi, both enzymes are responsible for breaking down Kynurenine into the less harmful metabolites.
A similar trend was bound in the mice’s lungs. IDO expression in pulmonary cells was significantly upregulated 2x at 2dpi and peaked 4x at 4dpir, reversing at 7dpi. KMO and KNYU were significantly reduced at 2, 4, and 7 days post-infection. The data so far points towards more KYN with an inability of the body to relate its catabolism (breaking it down).
This dysregulation could translate into a systemic one thus, they examined the blood of mice and humans. An increase in KYN (almost 4x), KYNA (6x,) and AA (anthranilic acid) (2x) was observed. This demonstrates what is observed in mice, is also observed in human patients, and points towards a systemic shift of Tryptophan metabolism.
Among its many functions, Kynurenine acts as a ligand for the extremely important Aryl Hydrocarbon Receptor (AhR). A ligand is something that binds to a receptor and initiates its many functions, and AhR is a transcription factor, meaning when it activates, it dictates which genes are turned on and off. Thus, many proteins are produced or not, it also regulates detoxification pathways, immune responses, the expression of other receptors, and responds to any non-organic structure (it was believed it was primarily a “pollution and toxin detecting receptor”).
As one would expect, that is what was observed in both the kidneys and the lung vasculature a reasonable increase of AhR was measured. The author’s previous work reported a new thrombotic axis, between AhR and Tissue Factor (a very potent activator of the coagulation pathway).
TF expression was upregulated in both lungs and kidneys. This means that in mice, this AhR-TF axis induced by SARS-CoV-2 infection causes inflammatory responses in the vasculature, contributing to a coagulatory state. To confirm these findings observed in mice, they analyzed both blood from SARS-CoV-2 infected patients and autopsy samples and confirmed these observations.
This paper confirms my observations that the Kynurenine Pathway and Tryptophan metabolic dysfunction contribute directly to a pro-coagulatory state, independently of the other myriad of ways the virus and its Spike Protein can also heighten coagulation. Kynurenine-IDO1-AhR is a self-perpetuating loop, one that has systemic and lasting consequences.
This pathway is an “immune tolerance” pathway, it is both immunosuppressive, and inflammatory, contributing to the long-term consequences of SARS-CoV-2 infection. Increased kynurenine levels are associated with post-stroke infection. Post-stroke infection significantly increases long-term decline and short-term mortality, and it can happen both in the first days, or even 76 days after a stroke.
The immune impression caused by the Kynurenine-AhR axis can reduce T-Cell infiltration, delay its immune response, and, as a byproduct, can aid certain infections to further reinforce the loop, such as Tuberculosis. Exposure to either LPS or Superantigens (SARS-CoV-2 core Neopolymorphic Toxin basis) outside the brain, can induce the expression of IDO in the brain.
These complex and often systemic cascade effects enable the infamous low-grade chronic inflammation, thus creating a very specific metabolic loop, the “Serotonin Shunt” pathway, a pathway that makes the body deficient in serotonin and induces state metabolic inefficiency in regards to Tryptophan.
Serotonin, as with other neurotransmitters and “neuro” hormones is produced largely in the gut and Serotonin is highly important in the gut.
Serotonin attenuates tumor necrosis factor-induced intestinal inflammation by interacting with human mucosal tissue
In the paper above, the authors uncover a highly significant pathway in which Serotonin modulates the immune system in the gut mucosa and has a potent anti-inflammatory action. It decreases the expression of chemokines, that call immune cells to a region of the body, here monocytes. Remarkably enough, the presence of serotonin and other acetylcholine, norepinephrine, downregulate the expression of TLR (TLR2, 3, 4, 6, 7).
Increased or dysfunctional expression of Toll-Like Receptors is found in all forms of infection, from acute, and severe to Long Covid, but especially lasting in the last two. A deficiency of serotonin could create a loss of this dampening effect, resulting in the upregulation of TLR, which leads to a dysregulated or exaggerated response against pathogens, viral fragments, opportunistic infection, and propensity to dysbiosis.
Continuous TLR signaling will perpetuate inflammatory loops, which will further drive inflammation, thus activating the IDO-AhR-Kynurenine pathway. Increased TLR expression in Long Cociv might not necessarily translate to better pathogen clearance. Instead, it might indicate a state of immune dysregulation where the immune system is overly sensitive and prone to maladaptive inflammatory responses.
Chronic neuroinflammation, driven by AHR activation or TLR signaling creating these immuno-inflammatory loops, can create an environment in the brain that is conducive to protein misfolding and aggregation. Inflammatory mediators, oxidative stress, and impaired protein clearance mechanisms in neuroinflammatory conditions could promote protein misfolding. This is more evident when enhanced serotonin function reduces Amyloid beta deposition.
Limiting inflammation and especially oxidative stress when infected with any respiratory virus, but especially SARS-CoV-2 will enable the body to curtail how lasting this shift towards the Kynurenine Pathway lasts, thus limiting the potential direct damage from microvasculature damage, enabling proper systemic function.
The best “Kynurenine Pathway” correcting intervention that exists is simple and free. Exercise. Proper sleep. Melatonin is a potent modulator that shifts the KP towards proper Tryptophan metabolism and certain vitamins, such as B1 (indirectly), B6, Whey Protein (assures proper tryptophan absorption), Taurine, Flavonoids, you know by now =).
Thank you for supporting my work or if you decide to start supporting it !








I may publish something else in the meantime, shorter, or longer, or something I feel like it, but the focus is on the Brain article now, which will take longer than usual.
I wish everyone a great weekend ahead.
Tuberculosis? And what do we have here?.... a tuberculosis outbreak... 67 cases, 2 deaths... in Kansas... (which is interesting as they moved the Plum Island biolab there.) And we have that new bird flu flying around Nevada cows -- D1.1. And Gates saying there WILL be another pandemic soon so we cannot do away with USAID or the WHO.. -- too late, old boy, it's done -- ... and, with the deep state in shock over the whirlwind taking place they may feel the only way to fight back is another pathogen release ... time to seriously stock up on health aids. How long do those peptides keep?