Today may be a two e-mail day of varying lengths. For the better part of the last two years and change, one of my lingering questions has been why or how SARS-CoV-2 fragments, pieces of the virus, are able to remain in the body for an absurd amount of time. Today’s article will be less “sciency” heavy in nature.
Antigen persistence, as in parts of pathogens lingering in the body for a brief period, is normal. One of the most common and lasting antigens in the body is from Influenza. Influenza fragments can last for weeks in the body, and it has been proposed that they aid the immune response and long-term protection against incoming strains.
But not all pathogens behave the same way, and not all lingering antigens are created equal. Persistent fragments from latent viruses like Herpes are known to trigger chronic inflammation and, as I explored in a recent article, can even push cells towards a misfolded protein response as a last-ditch protective measure.
SARS-CoV-2 EVs are somewhat understudied, but we have evidence for different effects, such as EVs carrying Spike Protein serving as decoys for neutralizing antibodies, a form of the virus that lowers our immune response. EVs from severe patients induce Lung inflammation, meaning when immune cells are exposed to SARS-CoV-2-derived EVs, they are able to induce a strong inflammatory response on their own.
“Treatment of bone marrow-derived macrophages with COVID-19 EVs enhanced the M1 phenotype and augmented the production of IL-1β, IL-6, and TNF-α.”
SARS-CoV-2 Spike Protein, SARS-CoV-2 Nucleocapsid Protein, and HIV GP120 Protein From Human BAL Are Contained Within Extracellular Vesicles and as Free Protein
INTRODUCTION:
We and others have previously described the persistence of SARS-CoV-2 Spike protein and HIV GP120 protein in bronchoalveolar lavage (BAL) fluid, but their origin and state has not been finely elucidated. The primary goal of this work was to determine whether these viral proteins persist as free proteins or are predominantly found in the protected environment of extracellular vesicles (EVs) within the BAL.
METHODS:
BAL was obtained from two sources. One included samples sent to the Clinical BAL Laboratory at Indiana University for evaluation of possible post-COVID lung disease. The second source of BAL was from HIV-infected subjects who had participated in prior studies at Indiana University. Acellular BAL was filtered through 0.65um syringe filter prior to EV and free protein separation by Ultracentrifugation (UC) and Size Exclusion Chromatography (SEC). Acellular BAL, pooled EV fractions, and free protein fractions were assayed for Spike and Nucleocapsid protein antigens using commercially available ELISA kits (Kerafast) and HIV samples were analyzed for HIV GP120 by Western Blot. Confirmatory western blotting for common EV markers CD9, CD81, CD63, and Syntenin-1 was performed.
RESULTS:
SEC yielded purer EV populations compared to UC. Analysis of SARS-CoV-2 spike protein in BAL fluid by ELISA after EV isolation demonstrated that 56% of Spike protein existed within EVs and 44% as free protein. Similar findings were seen for Nucleocapsid protein. In contrast, analysis of GP120 in HIV positive subjects on HAART therapy with undetectable blood viral loads demonstrated that while GP120 could easily be identified in EV fractions by western blot, most of the GP120 exists as free protein within the BAL fluid obtained.
CONCLUSION:
Spike and Nucleocapsid protein antigens persist in BAL fluid from patients with post-COVID lung disease up to two years after acute infection with a majority of spike protein and possibly nucleocapsid protein being contained within EVs. In contrast, we found that most GP120 existed as free protein, which we speculate may reflect continued low grade viral replication by latent HIV infection. We are currently exploring whether these persistent proteins (free and those in EVs) may serve as chronic antigens driving the chronic pulmonary diseases prevalent in both patients with post COVID lung disease and well controlled HIV infection.
For those unaware, BAL is merely flushing out fluids from the lungs using saline, and you analyze that fluid for whatever parameters, markers you wish to study. Exosomes containing both viral particles and Spike Protein have been studied since 2021, and in fact, it was one of Pfizer’s earliest selling points for its “lasting protection” where they found Spike Protein inside EVs up to 4 months.
You can imagine Extracellular Vesicles as smaller cells formed from your normal, big cells, it was primarily thought that they were used for intracellular communication, cells sending signals, proteins, lipids, nucleic acids, but they are also able to carry toxins. Over the past decade, with advances in molecular biology, we have discovered EVs can do much, much more.
This study finally adds evidence towards what I believe is the primary mechanism for Spike Protein persistence. Here, the authors found that EVs can carry both Spike Protein and Nucleocapsid protein even after 2 years, the samples came from patients with acute infections with likely lung disease, so these aren’t mild cases. It also measured HIV’s infamous GP120, important for our context here.
First, 44% of the Spike Protein and Nucleocapsid protein exist as free protein, meaning they are circulating around and can engage cells and initiate inflammatory processes, but 56% were EVs. GP120 remained mostly as free proteins, and this matters as much as the EVs.
EVs will protect their cargo from degradation by the body, especially if the EV is found within membranes or mucus, which allows this vesicle to avoid degradation and being “delivered” for even longer, but GP120 remaining a free protein, similar to over 40% of Spike Protein is as important, simply because the Spike contains 4 immunologically active, and toxic fragments from GP120.
In a rather paradoxical sense, EVs carrying viral fragments will act as time-delayed payloads, which will only activate when encountering a cell. And this mechanism can explain why some research groups found Spike Protein fragments inside the macrophages of Long Covid patients months after the initial acute infection, as Macrophages are known to take up EVs, and this will affect their function, and inflammatory signaling.
There is a secondary and tertiary mechanisms for Spike and fragment persistence, besides small fragments of the virus forming protein complexes, but I will leave this for another article in the future. EVs may also explain the rather absurd lag present after Omicron infections, where you can measure immunological changes even up to 10 months after initial infection.
In January, a peculiar and important paper was published, and I decided to wait to cover.
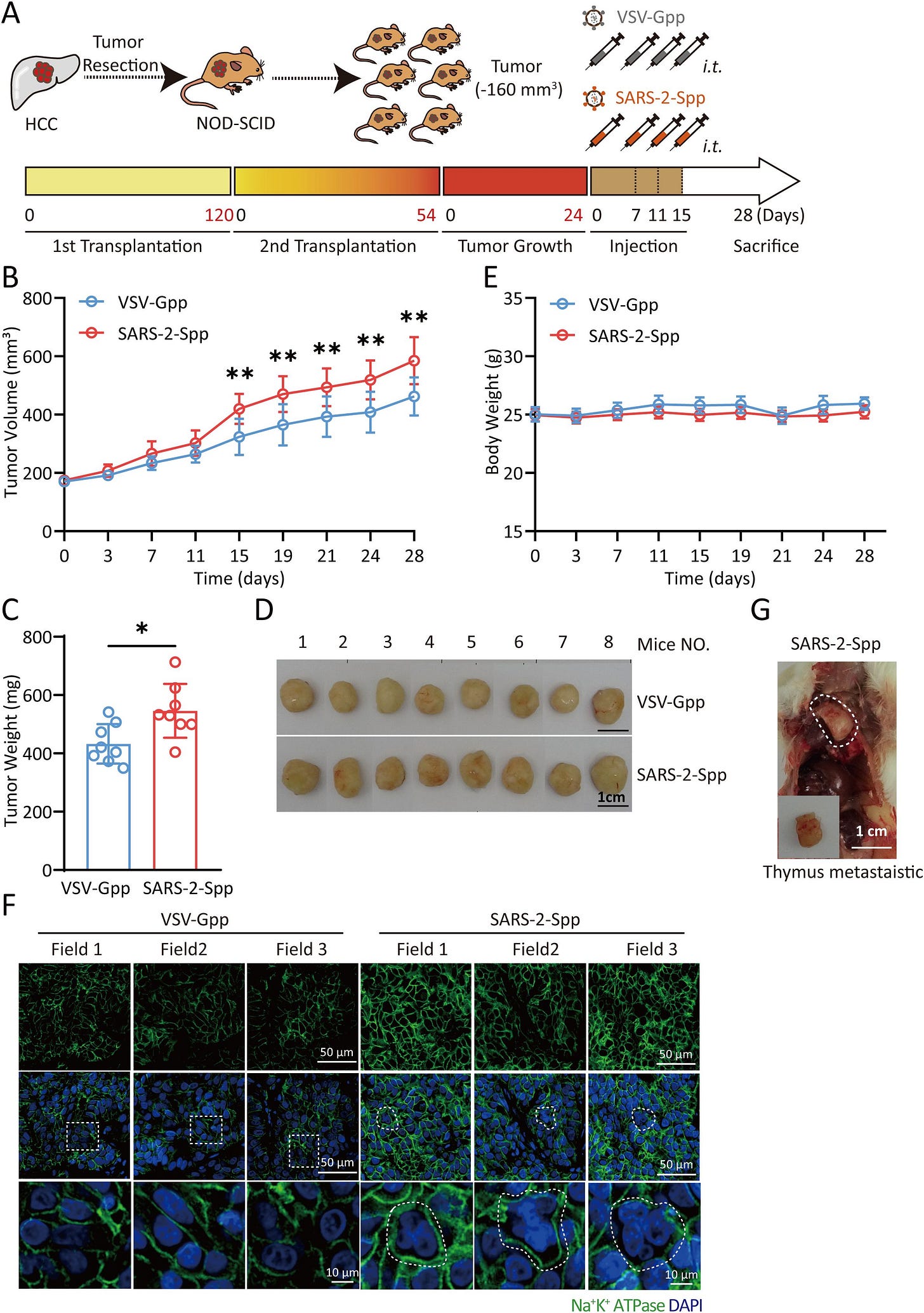
Exosomes derived from syncytia induced by SARS-2-S promote the proliferation and metastasis of hepatocellular carcinoma cells
In summary, SARS-2-S plays a significant role in tumors. Low-titer viruses such as SARS-CoV-2 use syncytia to escape the host’s immune defenses. These viruses continue to replicate and infect other cells to form additional syncytia. These syncytia continuously secrete exosomes, which are crucial in the tumor microenvironment. Cancer patients who do not develop inflammatory cytokine storms or ARDS in response to SARS-CoV-2 infection may still face a risk of rapid tumor proliferation and metastasis due to COVID-19.
We provide evidence that Syn-Exos induced by SARS-2-S can promote the proliferation and metastasis of hepatocellular carcinoma cells. Our findings reveal a new mechanism for the development of liver cancer in patients with COVID-19. In future studies, Syn-Exos could be used as targets for COVID-19 research. These findings could lead to treatments for cancer patients suffering from COVID-19. There are also broad prospects for developing related antitumor drugs based on this target.
Syncytia is basically the clumping of cells together, forming a bigger cell. During viral infections, viruses will use this effect to evade the immune system, as they use the first infected cell to fuse with healthy cells, thus avoiding the immune system long enough to replicate.
My Substack alone has over a dozen articles covering the roles of the virus and especially the Spike Protein on tumorigenesis and tumor acceleration, but here the effects are rather distinct. Syncytia induced by both SARS-CoV-2 and its Spike Protein induce the creation of Exosomes that will carry Spike Protein among other cargo and induce both the proliferation and metastasis (spread around other cells and tissue) of cancer in the liver.
It would be of the utmost interest of mankind to measure how much Spike Protein and other viral fragments remain in a healthy person after each “mild Omicron infection”, given that Omicron is the most immune-evasive coronavirus to ever exist (so far at least).
From a first principles approach, and marginally accurate napkin math, I would infer that a healthy adult would get rid of the vast majority of fragments, viral RNA over the span of 8 weeks, with any remaining fragments dealt with later on. How much fragment accumulates over time is my question.
Another one of my inquiries was the stealth nature of Omicron, different research groups have demonstrated different mechanisms by which this variant is able to evade the immune system, but the initial one remained rather obscure. Not anymore.
Stealth replication of SARS-CoV-2 Omicron in the nasal epithelium at physiological temperature
Here, the authors wanted to understand how the specific properties that made Omicron BA.1 a rather unique variant, many of the forthcoming variants post BA.1 were a byproduct or heavily affected by it, an evolutionary bifurcation if you will. Omicron variants are mostly URT infections, upper respiratory tract infections, they seldom reach the lung (although they have lung impact in many).
The authors used a different model than other groups, they reconstructed human nasal epiphelia, and with a rather interesting method. These cell cultures were exposed to two temperatures, 37°C and 33°C, with 33°C being the temperature in your nasal cavity.
Compared to other variants, Omicron had an earlier and more efficient replication rate, especially when the 33°C temperature was maintained, at this temperature BA.1 viral RNA reached near-peak levels in just one day-post infection, significantly faster than D614G (one of the most common variants used in many papers), at day 2, BA.1 RNA load at 33°C was significantly higher than the other variant.
Respiratory infections will often damage the nasal ephitelial integrity, and here they used a test called Trans-Epithelial Electrical Resistance (TEER) as a means to measure this. While at 37°C all variants demonstrated a similar decrease in TEER between 1.5 to 1.9 times, at 33°C BA.1 had stronger impact at 2.5x, Omicron is particularly more damaging to the nasal cells than other variants.
Cell damage may be accompanied by cell death, and by using LDH (Lactate Dehydrogenase) and activated Caspase-3, both markers for cell death, the damage trend was followed in cell death. BA.1 had a stronger cytophatic effect at 33°C when using LDH as a marker, with only Delta being similar at 33°C. When using Caspase-3, Omicron was the most efficient variant at both temperatures.
Have you met anyone with a lasting cough or irritated throat after an Omicron infection, that took significantly longer than usual to clear ? The likely answer is yes, and this was the authors next finding. Omicron impacted the motile cilia layer the most, and these are the cells responsible for cleaning mucus from your airways.
At 37°C, measuring at day 4 post-infection, all variants caused some cilia loss, but only Omicron caused significant loss, at 33°C, the cilia loss was overall smaller, but Omicron still caused a significant decrease compared to the control. Failure to clean mucus has a dual-edge characteristic, on one side, it may limit the spread of the virus, but on the other, it limits the clearance of viral particles trapped in the mucus enabling the virus and its fragments to penetrate deeper, but above this, inducing inflammatory and immunological changes that explain why Omicron is often followed by higher rates of bacterial and fungal infections.
Omicron’s stealth nature lies on 3 things, it replicates much better at nasal temperature, quickly and causes damage (including crippling the cilia-driven cleaning system) right at the point of entry (the nasal cavity), but does so while eliciting a weak early immune response in that specific temperature.
Thank you for your continued support !







I love it when my keyboard fails when writing THE TITLE.
A The Fourth Option article tomorrow or Sunday.
As an addendum separated from everything else, if you are experiencing memory or cognitive problems in recent moments, a significantly high dosage of Thiamine will help.
500 mg twice to four times a day. Yes, it is a lot, but the effects should be perceptible and quick. B1 has a peculiarly distinct kinetic in the human body.
Sleathy is an actual word lmao. -> https://www.wordsandphrasesfromthepast.com/word-of-the-day/sleathy
ETYMOLOGY
? from Old Norse slœ́ða to drag, trail (so Norwegian slöda; also, to work carelessly; cf. Old Norse. slóði (Norwegian slode) sloven, sluggard, whence perhaps. north-eastern Scotch sleeth