In memory of a dear friend of mine.
Both of the following Substacks are borderline must-read to understand how impactful what we are about to discuss here. Spike Protein as an endotoxin delivery system, and The Shift in mRNA Immunity.
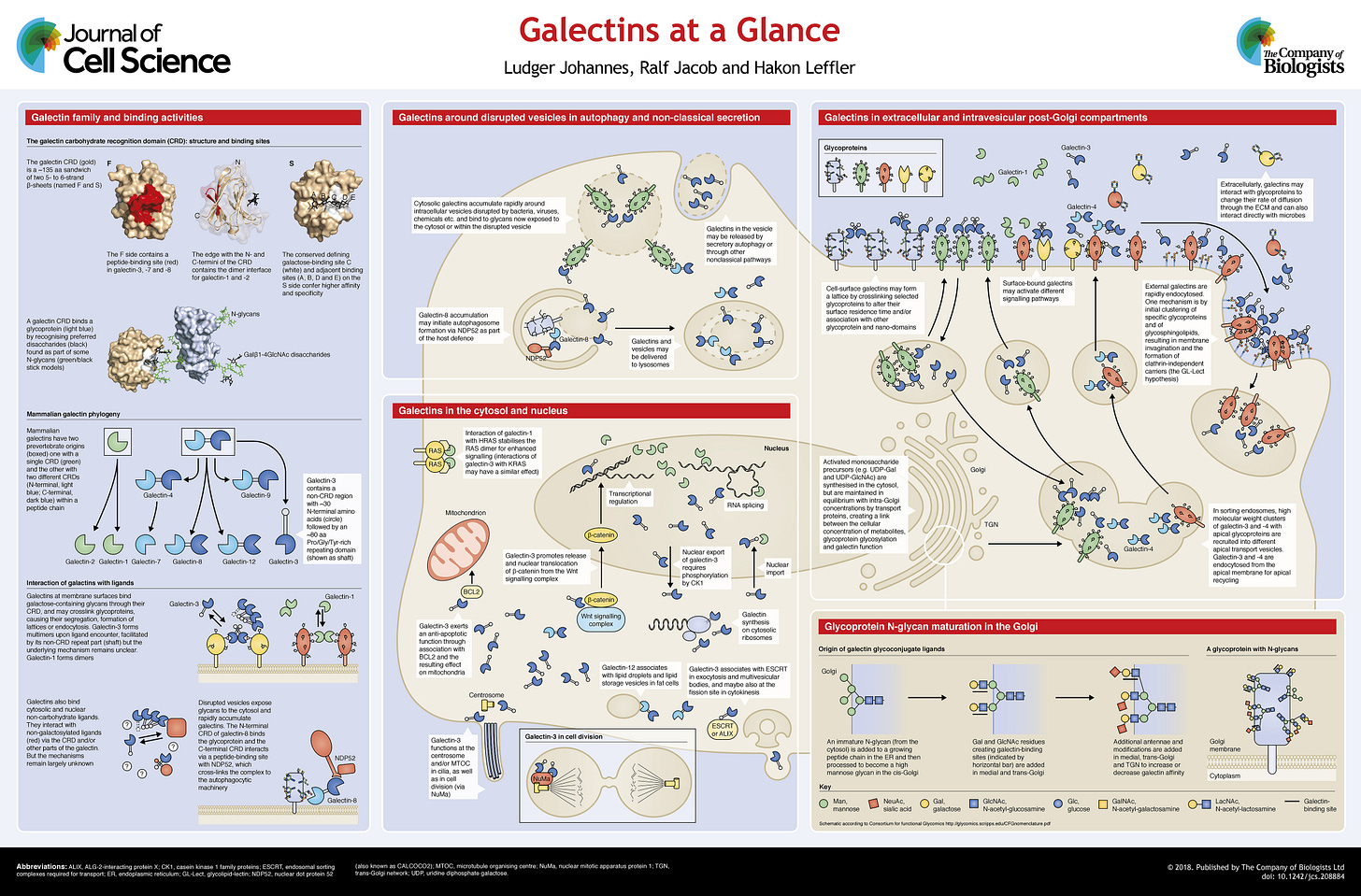
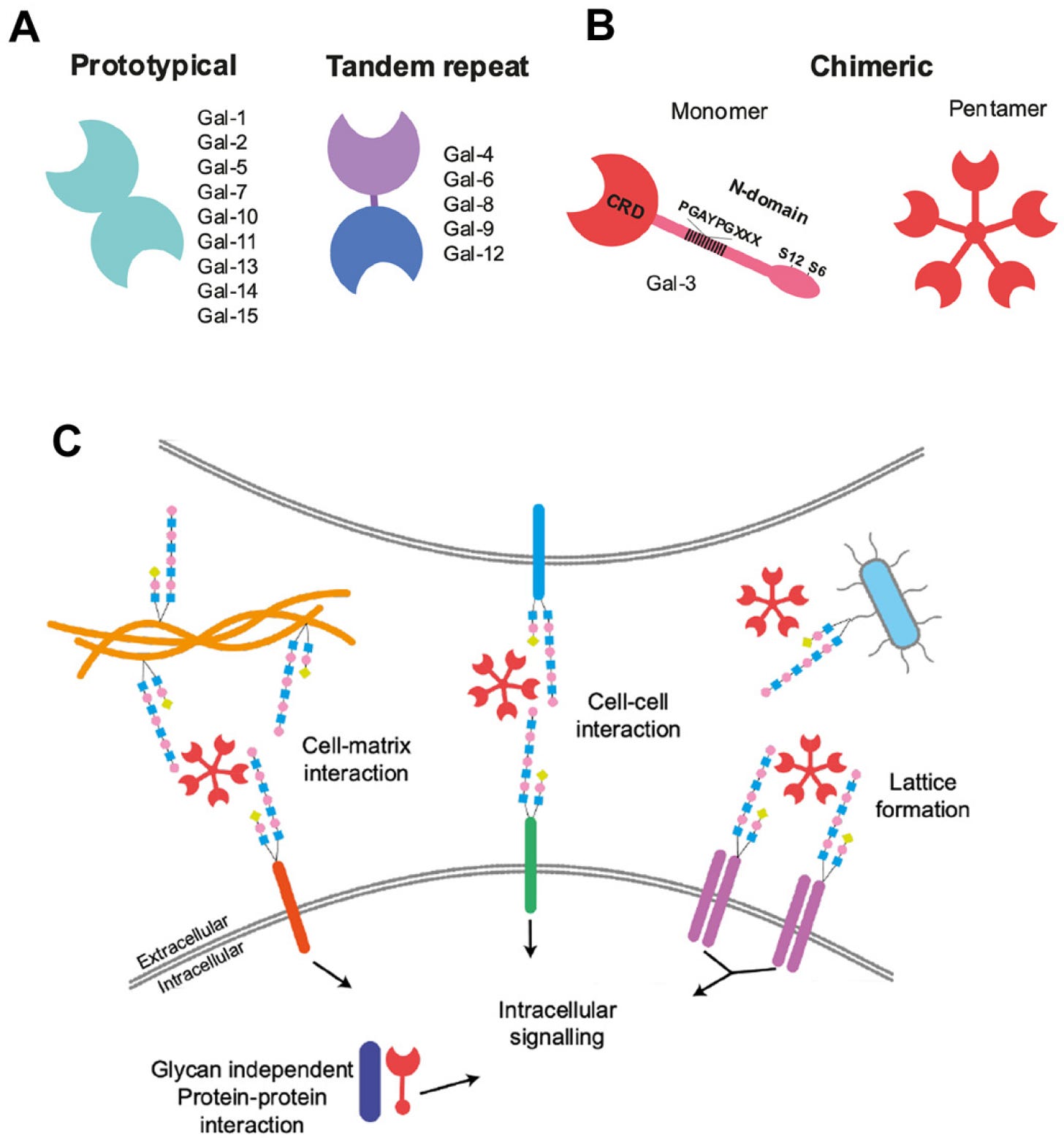
First, we need to clarify what are Galectins, before going any further.
Galectins are a class of proteins that bind specifically to β-galactoside sugars, such as N-acetyllactosamine, which can be bound to proteins by either N-linked or O-linked glycosylation. They are also termed S-type lectins due to their dependency on disulphide bonds for stability and carbohydrate binding.
Galectins are often referred to as “carbohydrate-binding proteins”, commonly synthesized in the cytosol (inside the cell liquid), and live most of their lives in the cytosol or nucleus, later it was found their Carbohydrate Recognition Domains (as the name implies the parts of the Galectin that recognize carbs) can interact with non-carbohydrate binding sites, this will be fairly important later on this text, and overall for the reader who goes into the Galectin rabbit hole, it is one of the most impactful aspects of Galectins, well one specific Galectin.
In a very simplified manner, these are proteins that primarily interact with carbohydrates, have quite an extensive number of cellular and physiological effects, but also interact with other types of molecules, beyond carbohydrates. Some Galectins are also involved in the splicing of pre-mRNA and in gene expression. Below is a representation of just a few functions these proteins can execute.
One may be pondering “so Galectins have a role in disease, and are related to carbs, why have you kept mentioning them in the last few weeks ?” Well, because Mother Nature (or a very long-term thinking designer…) has quite the sense of humor.
Role of the GTNGTKR motif in the N-terminal receptor-binding domain of the SARS-CoV-2 spike protein
The NTD of the SARS-CoV-2 contains a receptor-binding motif different from that of SARS-CoV, with some insertions that could confer to the new coronavirus new receptor binding abilities. In particular, motifs similar to the insertion 72GTNGTKR78 have been found in structural proteins of other viruses; and these motifs were located in putative regions involved in recognizing protein and sugar receptors, suggesting therefore that similar binding abilities could be displayed by the SARS-CoV-2 S1-NTD. Moreover, concerning the origin of these NTD insertions, our findings point towards an evolutionary acquisition rather than the hypothesis of an engineered virus.
In layman’s terms, the upper portion of the S1 (the part that interacts with cells to infect you) has a motif, a peptide sequence also found in other viruses and this motif closely resembles a Galectin, in this case, resembles it so well it was named “Galectin-fold”, because not merely the sequence but the shape mimics a Galectin. But not solely of peptides, a protein exerts function, so does this Galectin motif plays any role in regards to SARS-CoV-2 ?
The S1 Subunit of the SARS-CoV-2 Spike Protein Activates Human Monocytes to Produce Cytokines Linked to COVID-19: Relevance to Galectin-3
It is further known that the spike protein (S) of SARS-CoV-2 (as first reported for other β-coronaviruses) possesses a so-called galectin-fold within the N-terminal domain of the S1 subunit (S1-NTD). This fold (or pocket) shows structural homology nearly identical to that of human galectin-3 (Gal-3). In this respect, we have recently shown that Gal-3, when associated with epithelial cells or anchored to a solid phase matrix, facilitates the activation of innate immune cells, including basophils, DC, and monocytes. A synthesis of these findings prompted us to test whether segments of the SARS-CoV-2 spike protein might also activate innate immune cells in a manner similar to that observed in our Gal-3 studies. Indeed, by immobilizing S components onto microtiter wells, we show that only the S1 subunit (with the NTD) activates human monocytes to produce a near identical pattern of cytokines as those reported in COVID-19-related CRS. In contrast, both the S1-CTD/RBD, which binds ACE2, and the S2 subunit (stalk), failed to mediate the same effect. Overall, these findings provide evidence that the SARS-CoV-2 spike protein can activate monocytes for cytokines central to COVID-19, thus providing insight into the innate immune mechanisms underlying the CRS and the potential for therapeutic interventions.
Within the NTD of SARS-CoV-2 (and other β-coronaviruses) is a region often referred to as the “galectin-fold”, given its high degree of structural homology to that of human galectin-3 (Gal-3) (20, 21). Because of this remarkable similarity, it has been proposed that the S1-NTD of SARS-CoV-2 may very well act like Gal-3 and that this might explain, in part, the immunological sequelae observed in COVID-19 (22, 23). Indeed, intracellular Gal-3 has been linked to immune cell activation, namely that of monocytes/macrophages (24). We also recently reported evidence that epithelial cell-associated Gal-3 (EC-Gal-3) can activate a variety of innate immune cells to produce pro-inflammatory cytokines (25–27). In particular, we showed the activation of human dendritic cells (DC) and monocytes, demonstrating that these cells produced high levels of IL-6 and TNF-α –two hallmark cytokines in COVID-19-associated CRS (27).
Not only does the said Galectin-fold exerts functions, but it mimics one Galectin specific, Galectin-3. I will refrain from the origins talk of this “Galectin-fold” in some Coronavirusesm but I would like to bring to attention a specific angle of this Galectin mimic.
The protein sequence and GTNGTKR have homology in both amino acids and shape to the following sequence and shape of a well-known and used protein in the research of certain diseases and as a molecular clamp.
I bring this up because the paper above, and anything in relation to these “inserts” was buried on purely political motives and self-interest of the parties involved, it has been 3 years, and research must be done on these inserts because as you are about to see, they play a role on the disease progression and in my opinion, possible long-term damage.
Here it is a visual representation of why Galectin-3 is so over-represented in many diseases and inflammatory conditions.
Similar in its nature to AhR, Galectin-3 is a chimeric, promiscuous protein, meaning it can effectively bind to many other types of proteins playing a larger role in many diseases, and since it is a carbohydrate-related protein (with function based on said capacity to interact with sugar molecules) it has a close relationship with metabolic diseases.
It has a role to play in both obesity and impaired glucose homeostasis. Type 2 diabetics often possess a significantly higher level of Gal-3 than healthy people, it can disturb insulin signaling, and induce Beta-Cell inflammation and death (further progressing a prediabetic state into a Type 2, or worsening the glucose control of a T2D).

The role of Galectin-3 in relation to metabolism, especially glucose-related metabolic pathways is of utmost importance to many interactions and cellular responses present in viral infections. It is the initial kickstart of what a friend aptly named “Doom loops”, one of the many mechanisms in which the virus/Spike Protein dysregulates the body into a dysfunctional state.
Under a diabetic cardiomyopathy state, given Gal3 glycolytic nature, it will enhance the oxidative stress, lead to higher glucose levels and induce cardiomyocyte injury and cardiac injury. It is a marker and a big player in fibrotic states. G3 is one of the best markers recently discovered to measure the progression of heart disease.
Gal3 is a difficult protein to “pindown” its pathways because it is one of the most situational proteins in our bodies, its function will be defined by a myriad of other factors.
Gal-3 appears to be an important factor in the orchestration of CD8+ T cell activity and proliferation. Gal-3 promotes the formation of CD8+ T memory cells following exposure to antigen and prevents their apoptosis. Gal-3 deficient CD8+ T cells have shown a greater propensity to apoptosis. However, Gal-3 deficient mice have enhanced virus-specific CD8+ T cell response and better control of viral infection due to increased cytokine production by Gal-3 KO CD8+ T cells

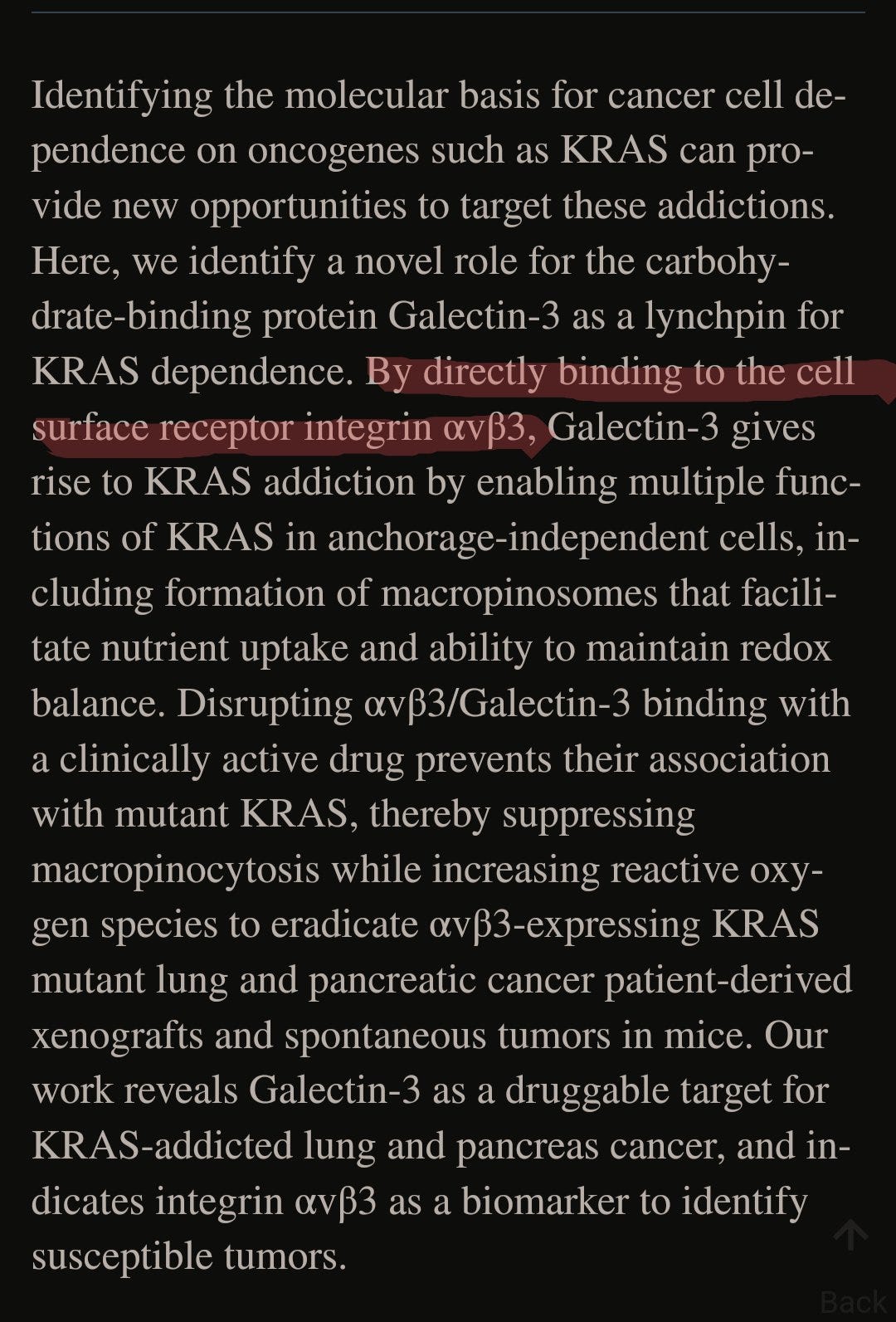
Galectin-3 has an extensive role in the tumor microenvironment, not only by influencing the secretion, and dynamics of your immune cells, but by directly affecting the expression of T-Cell Receptor. Upregulation of galectin-3 in tumors relies on transcription factors such as HIF-1α and NF-κB, and by now any of my readers should be aware of how large of a role HIF-1α plays in regards to many of the dynamics covered in this entire Substack so far, HIF-1α directly regulates Galectin-3 expression. And both of these are important factors in cancer, creating a positive feedback loop.
You can read about KRAS in relation to the mRNA vaccine in Genervter’s Newsletter. The S1-RBD part of the Spike Protein not only binds to αvβ3 and αvβ6 integrins, but is actively a selective agonist for these integrins, meaning it makes your body produce more of it. Integrins are another entire pathway that someone else covered, which I may cover in the future, they are incredibly important too. As a friend wrote, “modulating Galectin-3 would be a good way to target cancer, hypothetically”.
Gal3 will also affect Th17 differentiation, and in some cases, it will give you a higher Th17 protective response against fungal infections. IL-17 on the other hand is a key regulator between the interaction of Mucin and Galectin, playing a role in Asthma.
Gal3 directly plays a role in the Kynurenine Pathway, regulating IDO and exerting both inflammatory and immune suppression, affecting the course of the disease tested in the paper above.
Given how many functions inside, and outside the cell, by binding, and other dynamics and metabolic effects, it should be of no surprise Gal3 plays a role in Alzheimer’s disease and other neurodegenerative diseases, given the inflammatory nature of this protein. Galectin-3 levels are also correlated with Amyloid Beta and Tau aggregates in brain tissue in Alzheimer’s Disease, and there is a relationship between these levels and the early cognitive decline, memory, and synaptic dysfunction in this disease.
Galectin-3 can affect memory formation and fear responses via modulating Integrins. A good review of Galectin-3 role in neurogenesis, inflammation, and degeneration can be found here. Pharmacological Inhibition of Galectin-3 Ameliorates Diabetes-Associated Cognitive Impairment, Oxidative Stress and Neuroinflammation in vivo and in vitro
“Lastly, we herein show that Gal-3 expression is significantly increased in the frontal lobe of AD patients in parallel with enhanced Aβ oligomerization, and that the serum level of Gal-3 increases with the severity of memory loss in AD patients.”
Regulatory roles of galectins on influenza A virus and their potential as a therapeutic strategy
Highlights
•Galectins participate in regulation of influenza virus infection. •Galectin-1, Galectin-3 and Galectin-9 are key members that ameliorate IAVs infection and replication. •Antiviral capabilities of galectins against IAVs potentially occur through their extracellular and endogenous regulations.
It can execute either a protective or a harmful role during an Influenza infection, hedging on the strain and the preferred receptors that flu strain prefers to use, worth to note that its enhancing effects during H5N1 are very close to the same inflammatory response in the lung in SARS-CoV-2 cases.
In the Endotoxin substack, I wrote about the effects of Galectin-3 and LPS inside the cell, but here, LPS and Galectin-3 will also interact outside the cell, potentiating the inflammatory effects of a highly inflammatory endotoxin, via the canonical pathway.
By now the reader is aware of the many roles Galectin-3 plays in human physiology, and these are merely a few of the ones I decided to cover because they have a correlation with many of the pathways I have covered here, I could (if there is interest I might) write about its role in health, and many other aspects of other Galectins, such as 1, and 9, 9 being a sister to 3, and also playing different roles on different diseases.
Given its promiscuous nature, the presence of a Galectin-3 mimic in the Spike surface, the possible major role it plays, by forcing your body to modulate the expression of Galectin-3 via different mechanisms your body will often attempt to compensate, and this is where quite a few of the “after-effects” lasting weeks comes from in my opinion., at the very least in subsets of infected and vaccinated (mostly in the vaccinated though).
Given the relationship between G3 and IgG4 and the potential of the interaction between both to create the onset of different types of IgG4-related diseases, playing a role in mitochondrial dysfunction in this scenario too, it is of major importance to bring this to the awareness to more people, and at least for some researchers to look into the roles of the Galectin-fold, Galectin-3 levels in both the viral infection and vaccination (and obviously breakthrough infections).
And before I finish this, I must share another two aspects of Galectin-3, of major importance.
We detected a significant increase in corticosterone levels in both serum and thymus samples of galectin-3-deficient mice, as compared to age-matched controls. This was paralleled by an increase of gene transcription of the steroidogenic enzymes, steroidogenic acute regulatory protein (Star) and Cyp11b1 in thymus, 11β-Hydroxysteroid Dehydrogenase (Hsd11b1) in the adrenal, and Cyp11a1 in both glands. In conclusion, our findings show that the absence of galectin-3 subverts mouse thymus homeostasis by a mechanism likely associated to intrathymic and systemic stress-related endocrine circuitries, affecting thymocyte number, proliferation and apoptosis.
Two of the most concerning aspects of the Spike Protein and viral infection, often overlooked by many, is the damage it’s doing to HSCs, and here we have another angle when your body compensates for the fluctuation of Gal-3 in your body. Exhaustion of HSC is a fancy way of saying inflammaging, and immune dysfunction. (here is a substack about the Spike Protein damaging HSCs
And last, Gal-3 impacts thymic function. Something SARS-CoV-2 already affected.
Thank you and big appreciation for the supporters here and on Kofi, and also everyone who shares, also helpful !











If you than ad the 2nd cleavage site cathepsin.... Holy cow... So it is depending on epeigenetic preconditions which will be 1st striking: the more cancerous FCS or the more neuropathologic Cathepsin B CS....
Weird thing, dear John.
Just wow!
A brilliant tutorial on Gal-3
I know that there is a preprint on Gal-3 as a potential biomarker for severe COVID
.https://www.nature.com/articles/s41598-022-05968-4
All things cancer command my interest. The HIF-1 oxygen pathway upregulates Gal-3? I know about K-ras (the alluded to article was in German) . And, of course, there is the role of Gal-3 in Alzies
.I just learned about the interplay of the SPIKE with bacterial biomasses and the revelation of the LPSs. So, now, LPS and Gal-3 can interact outside the cell, potentially generating inflammatory effects? We can not win!
Thank you~ Time to study. Uh, oh, is this really just Part 1?
KINDLY READ:
Kindly read John Paul's comment on Twitter that I just saw-
"I don't know which is worse, the fact that Galectin-3 can bind to LPS and create a molecular bomb, or it creates an autoimmune reaction when you have a lot IgG4 around."