When Tau protein becomes a weapon against viruses
Our lasting enemy, Herpes viruses
This article will be more narrative, less science-heavy, and descriptive in nature. Even as early as 2020, there have been significant data, both from a classical clinical perspective and from a real-world data one on SARS-CoV-2 impact on systemic immunity, with some minor attention to what I would consider the chimera most significant impact. The reactivation of latent viruses, and while there are a few distinct families, the one I focused on the most is the one that is most widespread.
Herpes viruses.
Over time and with extensive research, I have argued that Herpes viruses and especially their reactivation dynamics plays a large and significant role in long-term damage, which was first observed in the clinical data of severe patients who developed pneumonia or sepsis. Increase in mortality is discussed among researchers, it does increase hospitalization, the amount of intervention necessary, but it does increase over time and, most importantly, complications. My focus was not the mortality but on the highly non-linear effects of the reactivation.
The mere process of reactivation has rather lasting immunological effects, where not only is there a sustained inflammatory surge, both local and systemic, but a shift in your whole immune system. And within our recent theme of proteins or fragments of pathogens lasting in the body, Herpes is another perfect example where one of its fragments can cause the entire body to shift towards a Th17 (inflammation that destroys tissue, and causes more changes), among many other immune-altering effects.
I will quickly summarize recent evidence on the role of SARS-CoV-2 in Herpes reactivation and what exactly that entails, with some of these previously mentioned last year.
In China, there was a significantly higher burden of Herpes Zoster reactivation during 2023, meaning Omicron variants (arguably milder pathogenity), the burden was higher comparing 1990 to 2023
Dental diseases play a significant role in post-COVID complications, and both SARS-CoV-2 and different Herpes viruses, such as EBV, HSV, and CMV, are known to aggravate periodontitis, inflammation of the gum, and can induce tooth loss and other long-term complications
In Russia, in this study, they found CMV being the most reactivated Herpesvirus. Interestingly enough, not only do inflammatory markers increase, but glucose too in those who suffered reactivation
Using a large data set, here the authors observed that patients who suffered Herpes Zoster reactivation had a significantly higher risk of major adverse cardiovascular events, acute kidney injury, and general renal function decline (I covered this paper last year)
This is a peculiar paper in which a HIV patient with their HIV under control, with CD4 semi-normal, but had Herpes viremia for up to 2 years after detection, which during this period got Covid, and later on developed tuberculosis
Additional reading with deeper analysis on what these dynamics entail.
Thus, the reason we are here. Throughout last year, we came to understand that “misfolded” proteins are significantly more complex and play other roles than just creating disease, mostly neurodegeneration. Amyloid proteins, even the “bad amyloid”, often possess parts of their structure and amino acids that are inherently anti-microbial.
Amyloidogenic proteins (proteins capable of forming the “misfolded/bad amyloid”) can work in conjunction to trap, kill, and clear out the body of different pathogens. As I stated in articles pertaining to this subject, misfolded proteins are likely a forgotten, last resort way to deal with infections that overwhelm primary defenses. Could other misfolded proteins behave the same ?
Phosphorylated tau exhibits antimicrobial activity capable of neutralizing herpes simplex virus 1 infectivity in human neurons
Not only have I quoted and read the author’s work throughout the years, but they themselves now propose a similar hypothesis, and now attempt to drive it further. Tau, a critical protein to stabilize neurons, maintaining the integrity of the nervous system at a cellular level, will only become “bad Tau” under the presence of Amyloid beta, it does so by the process of phosphorylation (named p-Tau from here on), adding phosphorus to normal Tau, and they argue this must be a tertiary mechanism for protection when a pathogen escapes Amyloid Beta, citing the dynamics observed in Herpes Simplex 1 infections.
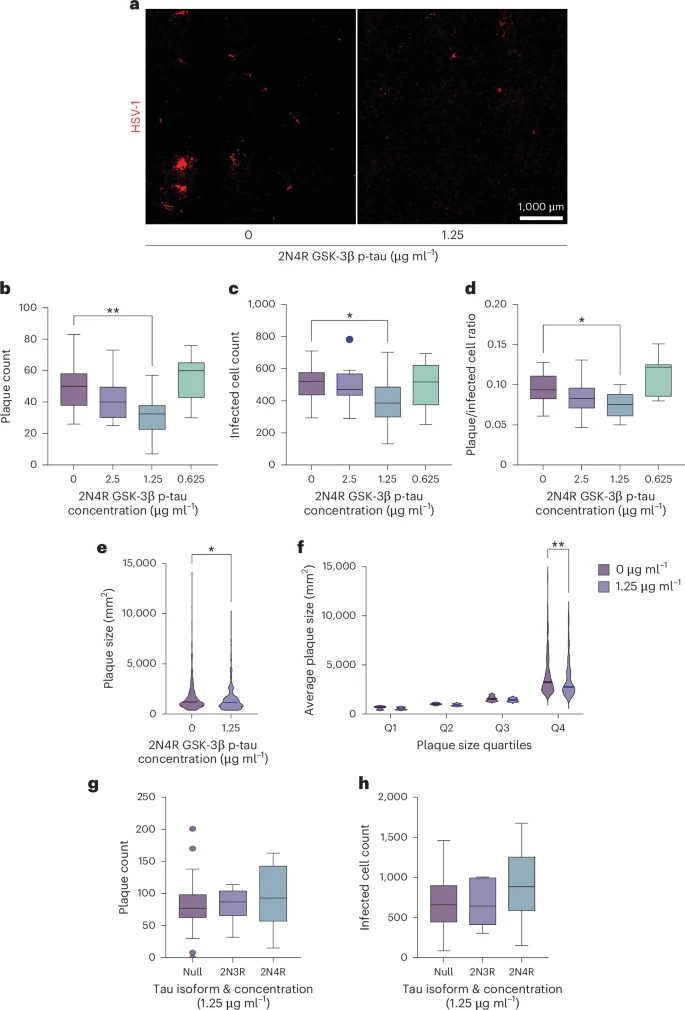
To test the antiviral potential of tau, the authors added p-tau to neuronal cells, and then proceeded to infect them with HSV-1. This confirmed p-Tau had an antiviral effect by limiting the herpes viral infection of the neuronal cells. Doing so attenuated HSV-1 infection at a single-cell level at an average of 40% and plaque counts, used to measure the concentration of the virus, dropped by almost 50%. p-Tau by itself acts as a rather potent anti-viral against herpes. Non-p-Tau had no effect.
To understand how p-Tau is able to prevent the virus from replicating, the authors tested the binding affinity of tau to isolated HSV-1 capsids (the protective layer around viral DNA) vs the whole enveloped virus (the entire virus). By using antibodies that bind to specific proteins from the virus, they were able to observe if tau was blocked from binding or not.
p-Tau significantly binded to the naked viral capsid rather than the whole enveloped virus, meaning tau works inside the cell, after the virus enters it, and has no lipid layer around it. By blocking other viral proteins in similar fashion, the authors identified that p-Tau binds to the VP16 and VP21/22a proteins of HSV-1, these proteins are located in the inner tegument (a structure between the envelope and the capsid of Herpesviruses).
These proteins are essential for recruiting motor proteins such as dynein and kinesin, which enable the virus to travel along microtubules, and by binding to those proteins, tau quite literally locks the virus.
Now here is one of the most significant aspects of this paper, glycosylation. The authors suspected the binding was based on sugar structures. To test this, they flooded the environment with a simple sugar (mannose). This completely inhibited the binding of p-Tau to HSV-1. This proves that p-Tau operates as a Lectin, a sugar-binding protein, similar to Amyloid Beta. It identifies the virus not by its DNA, but by the specific sugar patterns on its capsid surface. Remember this section for later.
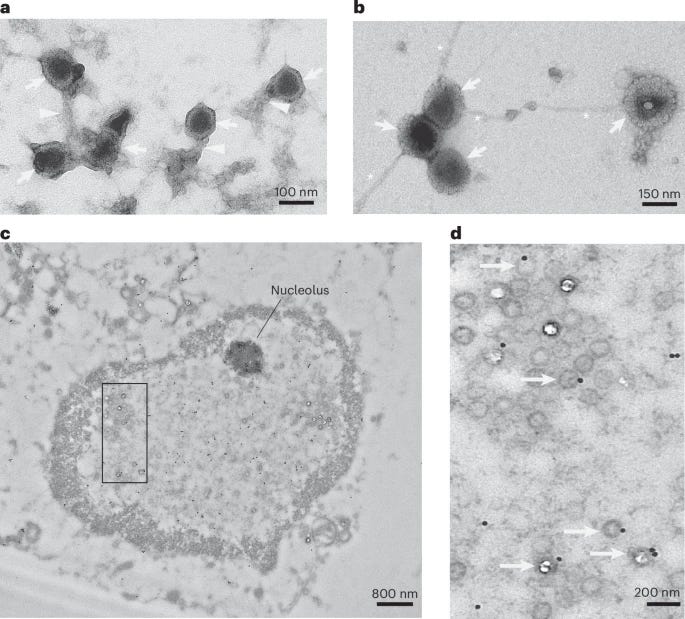
In immunological responses driven by antimicrobial peptides and proteins, these AMPs will often form net-like structures to entrap, kill, and clear off whichever pathogen they are fighting. To actually visualize it, electron microscopy was used, they mixed purified HSV-1 capsid with synthetic tau. The interaction caused tau to aggregate into fibrils with the herpes capsid, and this made the capsids physically clump (agglutinate) together.
These tau fibril-herpes aggregates are resistant to proteolysis and form stable virus-containing structures, thus limiting viral spread and infection of other cells. The following step was using HSV-1 and infecting 3D neuronal cultures, which are more accurate for understanding how neural responses occur in vivo. Infection of these 3D neuronal cultures led to both the aggregation of p-tau and also to the significant increase of p-Tau positive dystrophic neurites (damaged end of the neurons) and neuronal soma (the center of the neuron).
It was also observed an increase and a shift from soluble tau into insoluble, p-Tau, this shift into insoluble p-Tau correlates with the entrapment of the virus. After infection with HSV-1, intracellular soluble p-tau decreases almost 50%, while measuring p-tau outside the cell culture, experienced a significant increase in extracellular p-tau. Remarkably, this extracellular p-tau spread throughout the cells, and prevented the infection of other cells.
Historically, the cell-to-cell spread of tau (seeding) is viewed as the spreading of disease (like a prion). Here, the authors show that neighbors who took up the p-tau became more resistant to subsequent HSV-1 infection. This explains why TBI, sepsis, and other states where there is chronic HSV-1 reactivation cause widespread tau pathology, the brain is chronically activating this protective pathway.
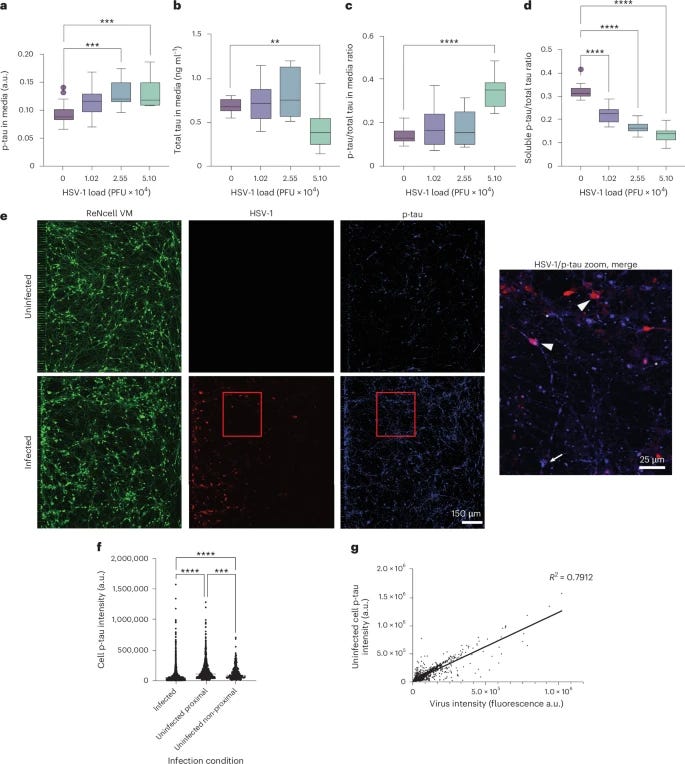
The last section of the paper is the most critical. Contrary to the view of aggregated tau as a DAMP (Danger-Associated Molecular Pattern) that inherently causes inflammation, the researchers found that adding synthetic p-tau to uninfected neurons did not provoke an immune response. It did not trigger the release of proinflammatory cytokines (IL-6, TNF, IL-1, etc.). This suggests that the uptake of p-tau is a silent, non-inflammatory physiological process intended to arm the cell without causing collateral damage.
The increase in p-tau inside the neighboring cells was driven by the physical internalization of the extracellular tau, not by an IFNγ signaling cascade. The propagation of the tau "seed" happens independently of the inflammatory status of the cell. When the researchers treated the cultures with anti-IFNγ antibodies (effectively removing IFNγ from the equation), the protective antiviral effect of the p-tau vanished.
For the p-tau to be effective, it must be properly internalized and trafficked to the correct intracellular location (the microtubule network or the nuclear periphery) to intercept the viral capsid. IFNγ signaling likely alters the permeability of the cell membrane or the activity of endocytic pathways, facilitating the uptake of the extracellular p-tau.
Without the background presence of IFNγ (which is naturally elevated during a viral infection), the neuron effectively ignores the extracellular p-tau, failing to internalize it or position it correctly, rendering the tau trap useless. Chronic neuroinflammation (mediated by microglia and IFNγ) and surges of IFNγ lead to the accumulation of these traps and eventually overwhelm the clearance system.
Lastly, the authors uncovered that microglia are responsible for identifying these tau-virus clumps and were able to observe microglia “eating” (phagocytosing) the tau-virus complexes, clearing the infection and the misfolded traps. Microglial dysfunction, glymphatic dysfunction are at the core of neurodegeneration, and this adds to the evidence on why. The clearance rate is overwhelmed, the body clears slower than the accumulation rate.
Real-world impacts, global effects
The findings in this paper are not merely adding to a greater body of literature on a real understanding of misfolded proteins and the roots of neurodegeneration. Post-mortem samples of Covid patients have widespread hyperphosphorylated tau in their brains, the Spike Protein causes synaptic dysfunction by p-tau aggregation and alpha-synuclein (and metformin protects against it). In a most recent paper on long Covid, the authors found an increase in phosphorylated tau in neurological LC.
Behaving like a carbohydrate/sugar binding protein is a major problem in regards to SARS-CoV-2. Not only does the infection often cause herpes virus reactivation, but the Spike Protein itself is also a heavy glycosylated protein, known to form a glycan shield around itself. This alone could likely create a reaction of the extracellular Spike protein interacting and forming a complex with extracellular tau.
But the real concern is the SARS-CoV-2 Spike Protein N-Terminal Domain, which contains a Galectin-fold, a sugar-binding protein that is shown to bind to hyperphosphorylated tau. This will form Spike-Tau complexes that will be internalized by cells via ACE-2 independent mechanisms, creating aberrant inflammation (IL-1β, IL-6), sensitizing immune cells towards tau pathology. This can explain neuroinflammation and neurological symptoms without direct brain infection.
The Galectin-fold has a multitude of cellular and immunological functions, and as it acts as a mimic, it can replicate what our Galectin-3 can do, but here, there is a more pertinent effect. The galectin-fold forms protein and RNA complexes called xenoAMPs, which, if proven to interact with tau (which I believe it does) it will form biomolecular persistent complexes that can drive neurodegeneration long after the viral infection is gone. It can also aid in the ACE2-independent entry via sialic acid binding.
And yet, most of the world thinks a surge in herpes reactivation on a yearly basis, induced by a virus that both induces an increase and accumulation of antimicrobial, proteolysis-resistant proteins and forms lasting complexes, is harmless.
Thank you for your continued support, and if you want to help my research, consider becoming a paid subscriber or buying my Kofi. It funds my research =).






This took much longer because I keep losing power, and in the last 3 days suffering almost a dozen brownouts. I will figure something out, invest on something to keep power running to work, because this is delaying my writing too much.
I can't afford to lose this PC because the RAM alone now is unaffordable. Before someone recommends a UPS, there are no consumer-grade UPSs in Brazil that can handle my PC, they all suffer the same flaw (they will turn themselves off with the PC if there is a power surge/brownout because they can't handle the wattage. (around 600w, a bit more sometimes, similar builds from others around the net experience the same)
Only commercial-grade UPS can handle it, and that costs almost as much as the PC lol.
Moriarty, thanks for this and good luck with resolving the infrastructure issues. Not fun.
This article reminds me of a couple of publications by A Midwestern Doctor that make me think DMSO could be an option to address the issues you raise here.
A Midwestern Doctor published an article in 2024 on DMSO and skin care, including a section on treating Herpes. When I get the occasional beginnings of a cold sore, I just put 100% DMSO on it and it's literally gone by the next morning:
https://www.midwesterndoctor.com/p/dmso-revolutionizes-skin-care-and?utm_source=publication-search
In 2024 AMD also published an article on the use of DMSO for brain injury including amyloidosis. From the article:
"One of the most well-known protein misfolding conditions (which sadly is has also been linked to the COVID vaccines) is amyloidosis, a challenging to treat condition where misfolded proteins are produced in excess, clump together in the body, and gradually fill up organs, increasingly disrupting their function. DMSO appears to have the ability to both dissolve amyloid aggregates and eliminate them from the body, and in all cases where it has been attempted, no adverse effects were observed (e.g., see this study). As a result, at least 40 studies and case reports have shown that DMSO can treat numerous types of amyloidosis."
https://www.midwesterndoctor.com/p/dmso-could-save-millions-from-brain?utm_source=publication-search
Internal DMSO really helped me back off the inflammation from long Covid. I use it now internally on the two days a week I don't take a peptide.