In 2022, there was a streak of acute hepatitis cases in children, who often needed to be hospitalized, and the cause remained unknown. Propositions to the cause were plentiful, but evidence was lacking. I stated it was SARS-CoV-2, with a potential for viral reactivation in the liver, leading to immune-mediated damage, basically the body attacking the organ because there are pathogens around. As time went by, and with mounting evidence… viral fragment persistence remained in my mind.
For clarity, although the infection, and injection leaves a deep imprint and scar in the body, reductive as this explanation is, “nothing” up until 2022 explained “everything”, meaning there isn’t a specific proposed mechanism for persistent dysfunction, or delayed yet severe reactions… unless parts of the virus and its RNA persisted for long in the body.
Why does this study matters ?
To preface the entire paper, this study adds a lot of nuance and yet highly important information to my own work, and to understand the deep impact of SARS-CoV-2 inflicts on the body. Many recently published studies have continuously mentioned the same “Our study indicates fragment persistence” or, in certain cases, viral persistence.
Such as the recent study on MIS-C, this one adds to the hypothesis that fragment persistence can lead to a delayed and incredibly severe reaction wherever the fragment finds itself. And in the absurdly large number of cases it doesn’t, you see subclinical damage over time, and fluctuation of biomarkers in specific organs (most commonly liver, pancreas, kidney…brain, but this one is difficult to test in the vast majority of settings).
I will interject again after covering the paper.
Characteristic immune cell interactions in livers of children with acute hepatitis revealed by spatial single-cell analysis identify a possible postacute sequel of COVID-19
For reference, throughout the paper, I will refer to AHUO, which stands for “Acute Hepatitis of Unknown Origin”. Also for reference, Hepatitis is just a fancy word for “liver inflammation”. This study used 12 children who presented with AHUO and had liver biopsies. The average age was 9.5 years old when they were hospitalized. The children had typical acute hepatitis symptoms and markers, such as elevated bilirubin, ALT, AST, and LDH, with slightly impaired coagulation. Although the coagulatory system is highly complex, a simple rule of thumb is, whenever your liver is significantly impacted by any form of injury, your coagulation will fluctuate a lot.
They were also tested for Hepatitis A, B, C, D, and E, and were negative, the same for adenovirus, which in certain cases are responsible for AHUO, both blood and liver tests were negative. Although 3 patients had positive IgG tests, meaning prior exposure, but no active involvement with the current acute hepatitis case (antibodies last long, so they don’t work for a test for active infections). EBV can also cause hepatitis, and the children were negative. Next, they tested the children for SARS-CoV-2 antigen, be it Spike or Nucleocapsid, and found it in 11 out of 12 children, but they did not found active, replicating virus.
Next part they did a histopathology test, to look at liver tissue under a microscope and observe any changes. All cases had a predominant pattern of liver injury, such as inflammation in the portal veins, hepatic arteries, and bile ducts (portal inflammation), and inflammation across the liver parenchyma (functional liver tissue) was observed in over 80% of the cases, or using simple terms, damage to the veins and the liver itself.
Inflammation is often followed by necrosis (cell death), which was about in the area connecting different portal areas and central veins, also in over 80% of the cases. Half of the patients had eosinophilic infiltration, meaning the presence of eosinophils (a white blood cell associated with allergic reactions). Less blood, damage to the tissue or the veins equates to more inflammation, which will inevitably lead to lots of cells dying.
All these findings confirm acute hepatitis of viral origin, but lacking typical features, suggesting an immune-mediated process, especially with the lack of a substantial number of children with autoantibodies (3 of the 12). To understand the immune-mediated mechanism behind it, the researchers used IMC, which combines imaging with mass spectrometry, enabling them to detect large number of proteins, 40 in their case, in tissue samples, at a single-cell resolution.
This method allied with bioinformatic processing aided by AI enabled them to determine the liver microarchitecture with high precision, and identifying immune cell subtypes and Liver Sinusoidal Endothelial Cells (LSECs, they line the liver and are critical to its function and immunity), which were Lyve1+, a marker used to make the distinction between these cells and other endothelial cells.
8 of 12 patients showed higher immune cell infiltration when compared to controls, and they are named in the image as “Immune_high”. In this group, there were significantly higher amounts of CD8 T-cells, Myeloid cells, and plasma cells. Other immune cells, such as CD4, T-Regs, B-cells, and granulocytes, were lower or non-significant.
Children in the immune_high group had significantly higher levels of ALT and AST, enzymes that measure liver injury and, here, the severity of hepatitis. There was a positive correlation between the higher severity of inflammation (the enzymes) and higher levels of CD8 T-cells and myeloid cells.
To analyze if the T-Cell infiltration was connected with immunological changes outside the liver (peripheral blood), they chose 5 patients and found a higher CD8/CD4 ratio (more CD8 cells than CD4), and the CD8 cells had effector function (capable of killing foreign pathogens or starting inflammatory processes). The CD8 cells in the liver had exhaustion markers, and in the immune_high patients, the changes outside the liver (in the blood) mirrored the ones in the liver, and some of these cells also had markers for being memory cells, indicating persistent immune response in the liver.
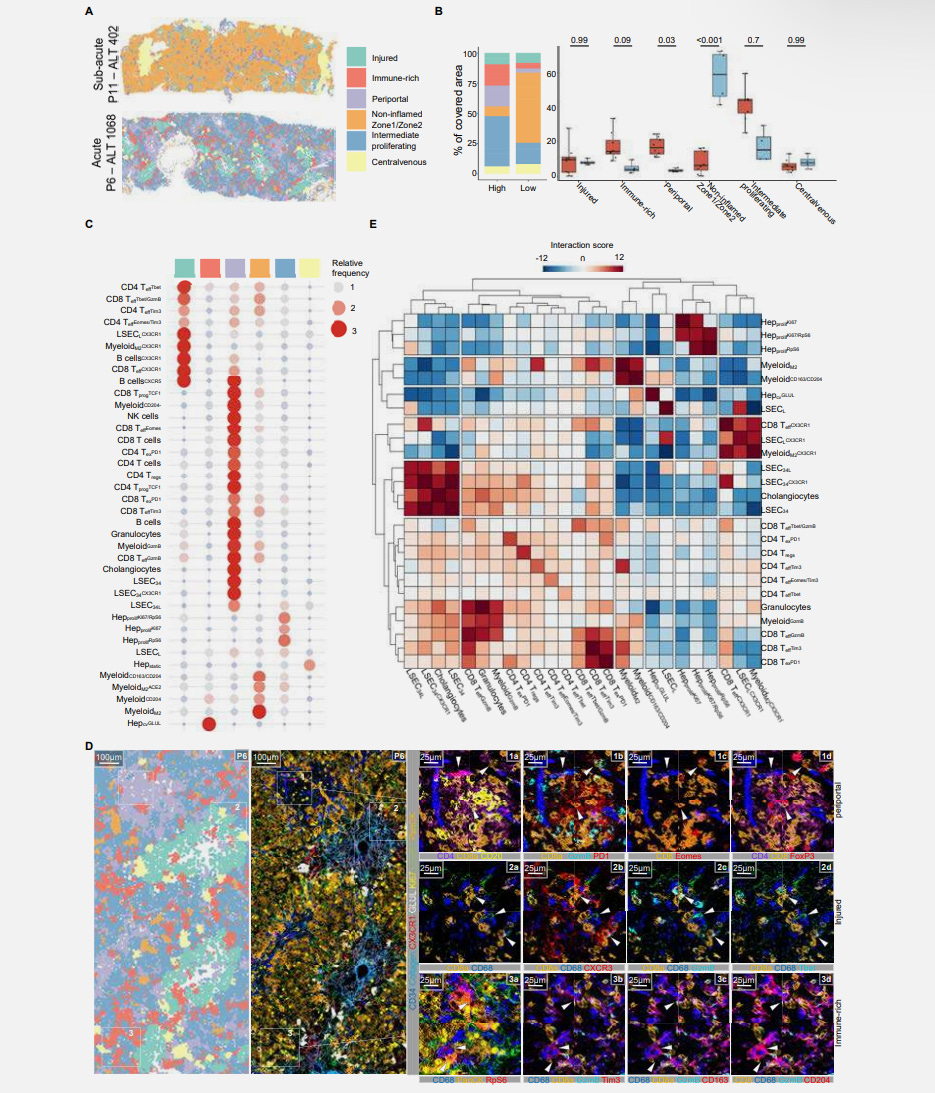
They followed up by using an algorithm called k-Nearest Neighbor (kNN), which you can use to identify the relationship and interactions between cells, their location, and which other cells they have more interaction with. They leverage the IMC data to do this step. And in this step, the authors had a breakthrough, they found specific groups of immune cells formed a pathogenic triad (I will explain the triad shortly), and this triad was associated with the areas that suffered injury in the liver (by collagen deposition, ergo liver fibrosis).
During their analysis, they found that regions with more damage had the presence of SARS-CoV-2 Spike Protein in cells expressing ACE2, and in fact Spike Protein formed aggregates with cells that expressed ACE2, LSECs, M2-like myeloid cells and hepatocytes were the majority of the cells positive for these aggregates, and they had the “pathogenic triad markers”.
This means that SARS-CoV-2 infects cells in the blood vessels of the liver, the tissue itself, causing the body to create a lasting immune response, with Spike Protein being able to be found in the tissue of the kids with AHUO, with a somewhat “allergic” (M2-like) immune response that is delayed, but extremely severe.
The Pathogenic Triad
The pathogenic triad uncovered by the authors is formed by the following - CX3CR1+ CD8+ T-cells, Liver Sinusoidal Endothelial Cells (LSECs), and M2-Myeloid cells, and I will break down exactly what this means.
CX3CR1 is a chemokine receptor, it is expressed in various cells but present in many of the liver cells (hepatocytes, LSECs and Kupffer cells, the liver-resident macrophages, part of the myeloid family), and its expression in immune cells is what tells the cells where to go (in this case, the liver). In this case, expression in CD8 cells means they have effector function (they can deal with a threat), but in specific conditions, such as chronic viral infection, or in this case, chronic stimulation from a persistent viral fragment, they can induce excessive inflammation and immune dysregulation.
LSEC, as we covered above, are the endothelial cells lining the liver, a form of specialized capillaries. They are structurally unique from normal endothelial cells, but LSEC can also act as antigen-presenting cells, presenting “Lego parts” of pathogens to T-cells. They are often involved in tolerogenic immune responses, attempting to mitigate inflammation, but in certain conditions, they become pro-inflammatory.
We recently covered myeloid cells, and M2-myeloid cells are another type of macrophage, often involved in tissue repair, and wound healing, lowering inflammation, and limiting fibrosis, they produce IL-10 (anti-inflammatory), and TGF-β (often immune suppressive), but in specific conditions, they will act paradoxically as promoters of fibrosis and inflammation.
The proposed mechanism would be that after a SARS-CoV-2 infection, viral fragments (antigens such as Spike Protein and Nucleocapsid) persist in the liver, and the antigens persist in the liver cells, and outside. Upon encountering them, LSEC and myeloid cells (these ones are responsible for “eating” harmful pathogens).
This activates these cells, in turn recruiting CD8 T-Cells to the liver, and since Spike Protein aggregates in some of the liver cells, these cells themselves become a target for CD8 T-Cells, which will kill them. LSEC damage can disrupt blood flow in the liver, thus promoting more inflammation, which in turn can affect hepatocyte function and viability.
This entire process damages the liver itself, which, per the author’s observation, of healing being preset at some level, this among other processes will initiated macrophage polarization into M2 type, as means to mitigate the inflammatory process, and heal the damage itself, but paradoxically here, it initiates a feedback loop exacerbating inflammation, causing a delayed, yet very severe and aggressive case of unknown hepatisis.
This article is already complex and layered, so I will be ending my analysis here, but the evidence presented here aligns with one of my proposed ideas, and proposed by the authors themselves, that these AHUO share many similarities with MIS-C, and represent a subset of PASC-like sequelae. MIS-C and AHUO shared the “delayed but aggressive” response aspect, while all three share the viral fragment persistence.
This is where the Viral Afterlife article comes in. Here, the authors unveils a mechanism where components of the virus itself, even after proteolytic (protein being broken into smaller parts) breakdown, don't just become inert debris. Instead, they can mimic potent host immune molecules (AMPs) and self-assemble with nucleic acids (released from damaged host cells, which happens in any viral infection) into surprisingly stable, nanocrystalline complexes.
These viral peptide-nucleic acid complexes aren't just floating around, they possess the specific structural properties required to activate innate immune receptors like TLR3 and TLR9, receptors usually tuned to detect pathogens. The activation isn't subtle, triggering a vastly amplified proinflammatory response in vitro and driving immune cell recruitment in vivo in mice, mirroring the systemic inflammation seen in severe infection.
The persistent SARS-CoV-2 antigens found in the AHUO patients livers, particularly in areas of damage, could be the source material for these proinflammatory viral peptides. Nucleic acids, released from liver cells undergoing necrosis and inflammation (features prominent in the AHUO), would be readily available partners for these complexes. These self-assembled viral complexes, acting as potent triggers for innate immunity via TLRs, could then provide the sustained molecular signal necessary to recruit, activate, and perpetuate the damaging activity of the pathogenic triad (CX3CR1+ CD8+ T-cells, LSECs, M2-Myeloid cells) observed in the AHUO livers.
Consider becoming a paid subscriber, and if you are one, thank you ! Or buy me a coffee.







The paper also hints at other pathways, such as TIM-3, although it does not mention TIM's favorite friend, Galectin-9. Also mentions and compares this acute hepatitis of unknown origins with acute anemia, which seems as a severe byproduct of myelosuppression.
I now have a few ideas on why fragments are lasting too long in anyone (injected will OBVIOUSLY last longer, and have more of lasting fragments).
Sugar is indeed the real killer.
I wish everyone a good rest of the weekend.
Sugar: I notice my sugar cravings go through the roof whenever a virus is attempting to make me its hostess. If I give in to sugar cravings, the virus gets a boost and my immune system takes a hit. If I manage to eat without spiking blood sugar, the infection will not get upper hand in my body. Sugar is dangerous.