A rising trend, both scientific, clinical, and linguistic (convergence) in the last 6 months has been “microclots” or “microvasculature alterations”, meaning damage to the very small blood vessels in our body, and our body and organs rely on these for proper function. Alterations in these very small veins for the vast majority of the time are not fatal, but they increase symptom burden, and dysfunction, given a long enough time, will cause disease. A perfect, poster boy example — Alzheimer’s Disease, and diseases in its class.
In May 2023 I covered an interesting paper.
Per the image, my analysis and the data, vaccinated people have a higher risk of all forms of RVO even 2 years after vaccination, and the unvaccinated still have the risk, but lower, this was back in the day. I will cover two papers briefly, with the third one being the most important and covered in length.
Long-Term Effects of COVID-19 on Optic Disc and Retinal Microvasculature Assessed by Optical Coherence Tomography Angiography
Results: The COVID-19 group showed lower VD values than the control group in the nasal parafoveal quadrant of the SCP at all visits (p = 0.009, p = 0.47, p = 0.042) and in the superior perifoveal quadrant of the DCP in the twelfth-month visit (p = 0.014). At all visits, FAZ area and FAZ perimeter were higher (p = 0.02, p = 0.02, p = 0.002; p = 0.002, p = 0.003, p = 0.005), foveal VD values of both SCP and DCP were lower (p < 0.001, p < 0.001, p < 0.001; p = 0.005, p = 0.001, p = 0.001), and CMT was lower (p < 0.001, p = 0.001, p = 0.001) in the COVID-19 group. The COVID-19 group had higher temporal quadrant RPC at all visits (p = 0.003, p = 0.003, p < 0.001) and higher average, superior and inferior RNFL at first and fourth-month visits (p = 0.014, p = 0.020; p = 0.001, p = 0.003; p = 0.021, p = 0.024).
Conclusions: There are long-term changes that mainly point to the ischemia in the COVID-19 patients. We emphasize the need for long-term ophthalmologic and systemic follow-up of COVID-19 patients regarding potential complications.
The vaccinated people in this paper were vaccinated using CoronaVac, not mRNA a significant, and important distinction, and the focus of this paper, unlike others covering “eye damage” is microvasculature. Also, the patients here are relatively young and have mild to moderate infections. And thus one of the conclusions:
“Furthermore, OCTA imaging results in COVID-19 patients corroborate the concept that the pathogenesis of COVID-19 disease includes a microvascular component.”
OCTA is a test designed to assess quite well microvascular alterations. Here the authors found long-term changes in the ocular microvasculature leading to a poorer blood supply, which in turn lead to long-term effects in the eyes. The next paper is quite nice because it is a review paper, these usually cover the why’s, how’s, etc.
Impact of COVID-19 on Ocular Surface Health: Infection Mechanisms, Immune Modulation, and Inflammatory Responses
One of the most common “ocular” effects of SARS-CoV-2, varying by variant (certain mutations have a more “eye-catching” effect than others) is conjunctivitis, symptoms are redness, itching, tearing, and increased liquid discharge. Dry eye is another frequent symptom when it feels like your eyes have sand grains inside them. Persistent dry eye may be linked to persistent inflammation and long-lasting immunological effects. Light sensitivity and eye pain are other ones, and blurred vision too (had all of these at some point lmao, I personally think blue light is a massive contributor).
I have long stated that the eyes are a vector of entry and infection and circumstantial, and overlooked epidemiological evidence can attest to that, it is not as common, but it happens. I have stated using evidence that the virus uses your eye nerves to attempt to invade the brain, causing neurological effects and inflammation, both at very low levels but contributing to pathology as years go by.
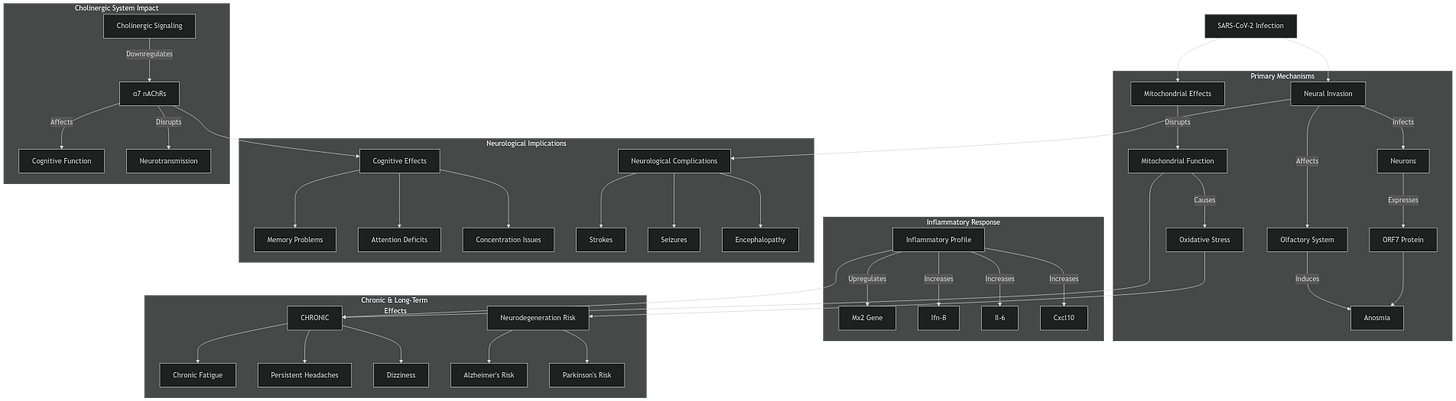
Ocular damage has many pathways. Chronic inflammation or chronic immunological response to viral fragments not present in the quote above will most likely be the causative agent when it comes to the long-term effects (the lag time between infection, and minor eye symptoms, followed by 12+ months later of perceptible ocular degeneration).
While primarily linked to pulmonary and systemic damage, these elevated cytokines—especially IL-6, IL-1β, TNF-α, and IFN-γ—can also profoundly affect the ocular surface Vascular dilation and increased permeability lead to tissue edema and conjunctival hyperemia, manifesting clinically as redness, swelling, and increased tearing. Excess inflammatory mediators and proteolytic enzymes may further damage corneal and conjunctival epithelial cells, weakening barrier integrity and predisposing patients to corneal ulcers, perforation, or secondary infections.
Complement activation, particularly the release of C3a and C5a, further heightens local immune responses, contributing to the microvascular injury associated with advanced disease states [93,94]. The net effect of these feedback mechanisms is often persistent ocular surface damage, potentially resulting in chronic inflammation, fibrosis, and vision impairment. Mitigating such systemic-to-local propagation of inflammation is essential for reducing the risk of long-term ocular complications.
In my opinion, based on the quite large amount of research I have done, the ocular dysfunction and long-term damage is a byproduct of the systemic effects of an infection, especially in regards to changes into microvasculature. Direct infection of the eyes will happen and certainly will aid in the microvasculature effects, and damage by direct infection and immune response.
Now to the last, and to me most significant paper. If you haven’t read me for long or read just articles that interest you, you may not know that after the brain, pancreas, and liver are the organs I am mostly interested in. As a reminder, this was the start of the microvasculature trend in SARS-CoV-2 research, microvascular damage in the kidneys, impairing Vitamin D metabolism (locally).
SARS-CoV-2 Spike S1 subunit triggers pericyte and microvascular dysfunction in human pancreatic islet
The COVID-19 pandemic has profoundly affected human health, yet the mechanisms underlying its impact on metabolic and vascular systems remain incompletely understood. Clinical evidence suggests that SARS-CoV-2 directly disrupts vascular homeostasis, with perfusion abnormalities observed in various tissues. The pancreatic islet, a key endocrine mini-organ reliant on its microvasculature for optimal function, may be particularly vulnerable. Studies have proposed a link between SARS-CoV-2 infection and islet dysfunction, but the mechanisms remain unclear. Here, we investigated how SARSCoV-2 spike S1 protein affects human islet microvascular function. Using confocal microscopy and living pancreas slices from non-diabetic organ donors, we show that a SARS-CoV-2 spike S1 recombinant protein activates pericytes — key regulators of islet capillary diameter and beta cell function—and induces capillary constriction. These effects are driven by a loss of angiotensinconverting enzyme 2 (ACE2) from pericytes’ plasma membrane, impairing ACE2 activity and increasing local angiotensin II levels. Our findings highlight islet pericyte dysfunction as a potential contributor to the diabetogenic effects of SARS-CoV-2 and offer new insights into the mechanisms linking COVID-19, vascular dysfunction and diabetes.
Highlights
• Different components of the renin-angiotensin system are expressed by vascular cells in human pancreatic islets.
• The islet microvasculature is very responsive to vasoactive angiotensin peptides. • This pancreatic renin-angiotensin system is targeted upon incubation with a SARS-CoV-2 spike recombinant protein.
• SARS-CoV-2 spike activates pericytes and constricts capillaries in human islets.
• Islet vascular dysfunction could contribute to dysglycemia in some COVID-19 patients
A common, but understudied effect of SARS infection (both the first and the second one) has been its “diabetogenic effects”, causing a dysfunction in how the body handles glucose (sugar) metabolism. Even to this day, there is discussion if and how the virus could actually infect the cells in your liver, the pancreas ? Well….
In a similar fashion to the Kidney microvasculature paper, here the remarkable finding was the virus doesn’t affect the function of your pancreatic cells responsible for generating insulin, but rather the S1 part of the Spike Protein affects the microvasculature, and following the renal trend, it impairs oxygen, nutrient delivery affecting glucose/sugar regulation.
First, the author had to assess if the pericytes (the cells around the walls of blood vessels) are potential SARS-CoV-2 since they do express ACE2. They measured ACE2 distribution in different areas of the pancreas and found ACE2 is present not in insulin-secreting beta cells (the most important cells in the pancreas) but predominantly in the perivascular regions. Markers such as Neuron-Glial Antigen 2 (NG2) and Platet-derived Growth Factor Receptor Beta (PDGFRβ) confirmed these ACE2-expressing cells as pericytes.
To confirm the observations above, they used single-cell RNA sequencing (scRNA-seq) which showed higher ACE2 transcript levels in pericytes compared to other cell types within the islets. Interestingly, ACE2 expression was higher the older the person is, pointing towards an age-related increase in ACE2, which would explain why older people and people with comorbidities died at such larger numbers in 2020, and why these are more susceptible to microvascular dysfunction.
ACE2 is a very important part of the RAAS (Renin-Angiotensin System), a hormone system that regulates blood pressure, fluid levels, electrolyte balance, and vascular resistance (thus controlling blood pressure). ACE2 counteracts ACE, by degrading the vasoconstrictor angiotensin II (AngII) into vasodilator angiotensin (1-7). To test if this occurs in the islet capillaries, the authors used Fluo4-AM, which helps to measure calcium levels ([Ca2+]i) in the pericytes, and this indicates their activation.
Adding exogenous AngII significantly increased ([Ca2+]i) in the cells, showing strong activation. Adding Ang 1-7, which is processed from AngII by ACE2 caused a minor reduction of ([Ca2+]i), showing the antagonistic role against AngII. Imaging showed that AngII-induced pericyte activation was accompanied by capillary constriction, with diameters decreasing by 8%. The decrease was confirmed by using losartan in the cells, an AT1 receptor antagonist, which abolished the constriction effects. This demonstrates the existence of a functional RAS in the human endocrine pancreas, and that islet vascular cells express its components (ACE, ACE2, AT1), and respond to the modulation of vasoactivity, thus affecting microvascular function.
With a functional RAS observed in the pancreas and its islets at a microvascular level, the next step is testing what SARS-CoV-2 Spike exposure causes, because Spike interacts with ACE2 as its primary form of infecting cells. They exposed slices of the human pancreas to recombinant Spike S1 (only half of the Spike). As control, they used HCoV-OC43 which is incapable of binding to ACE2. Fluorescent imaging showed that exposure to Spike S1 led to a sustained increase in ([Ca2+]i) in pericytes. The more Spike is introduced, the higher the calcium levels.
Exposure to Spike S1 diminished how pericytes respond to AngII, as observed by the reduced calcium response (and faster peak fluorescence). This indicates that the Spike Protein, especially S1 modifies how pericytes respond and how the capillaries function, likely by altering ACE2 activity or localization (internalization of receptors as a defense mechanism). Response to other vasoactive molecules such as Norepinephrine and Endothelin-1 were slightly reduced, while the inhibitory effect of Ang1-7 amplified, thus showing Spike alters their capacity to respond to different vasoactive molecules.
Since pericytes control and regulate capillary diameters, the author’s next step was investigating the effects of Spike S1 in the pericytes and whether it resulted in capillary constriction. Imaging showed that islets exposed to Spike S1 had significantly narrow capillaries compared to the islets exposed to HCoV. Adding extra AngII did not lead to further capillary constriction likely because they were already significantly constricted when exposed to Spike S1. However, adding Ang1-7 elicited potent vasodilatory effects. Exposing vascular cells in islets to Sars-CoV-2 S1 affects their function, makes them contract, and interferes with how they respond to other vasoactive RAS peptides.
As I mentioned above, the process of the Spike interacting with ACE2 leads the body to compensate, thus the authors sought to understand the mechanism by which the Spike induces microvascular dysfunction, and found precisely what I stated. When cells are exposed to Spike S1 they internalize ACE2, while remaining in the plasma membrane (outside) in the control. Measuring AnII in the Spike-treated cells further confirmed the loos of ACE2's ability to degrade AngII into Ang1-7. These results provided a mechanistic understanding of how SARS-CoV-2 disrupts and affects microvascular function in the islet.
Finally, the study examined whether the observed pericyte and vascular dysfunction affected beta-cell insulin secretion. Despite the severe vascular changes, glucose-stimulated insulin secretion remained unaffected in spike-treated slices after one hour of incubation. This outcome was surprising, given the essential role of pericytes in supporting beta-cell maturation and function. The metabolic dysfunction after SARS-CoV-2 infection is most likely an impairment of the functional vascular network, although there is significant evidence of the endocrine effects of the virus, complementing the endocrine disruptions reported in other studies.
To make my point about why this is highly important information, SARS-CoV-2 will induce microvascular dysfunction not just by changing how the body “uses” ACE2, but by a myriad of mechanisms, with this being the most significant one because Omicron didn’t lose its love affair for ACE2. This also explains the 3 to 6 months long changes in coagulation.
Sadly modern medicine is not equipped to deal with and track subclinical damage and is especially ill-equipped to diagnose microvascular alterations, it takes a keen eye (no pun intended) and specialized knowledge, but strides have been made in the last 2 years in this regard. Minimizing inflammation, and aiding recovery to the best of your ability should limit these effects in most people, but now we have mounting evidence that this has been and still is a “micro” disease. Death by a thousand cuts. And in response to this, you should consider Red Light Therapy, because…
Exposure to Red Light, therefore Red Light Therapy modulates platelet function and regulates thrombus formation, thus Red Light lowers your risk of forming clots and lowers inflammation. You should consider building a small red light exposure system in your room, or in a room in your house because it will greatly benefit your health. Not in the image but from here. I use a blue light-filtering lens myself because my monitor is big and it literally hurts my eyes…after using it for a while you literally can perceive the excessive blue tint in every screen.
They discovered that cancer patients who received blue light-filtering lenses had a lower risk of blood clots compared to their counterparts with conventional lenses. This is especially notable because cancer patients have nine times the risk of blood clots of non-cancer patients.
The next article will be about the brain, misfolded proteins, Herpes virus, the Glymphatic System, and by sheer coincidence (or competence) I was right again.
I am grateful for your continued support, and if you decide to become a supporter, you have my thanks. It enables me to continue researching.










I am still behind on messages, e-mails, and everything. Be patient lol.
Also I wish you all a great Sunday and great week ahead.
I got optic neuritis in one eye during the timeframe when there was massive Covid vaccinations being done. I was not vaccinated and hadn't had Covid before or during that time. Despite submitting to numerous tests and one brain scan, the cause was never found. To this day, I believe it was from vaccinated people shedding.