SARS-CoV-2 lasting impact in the bone marrow
Myelosuppression, Inflammaging
I often try to stick to a pseudo-schedule and otherwise “theme-centered” articles, covering specific aspects, but fate has the last laugh sometimes, thus, specific subjects jump into the priority list. While sticking to particular aspects, my actual research and notes will often resemble the training data and the layers of a Machine Learning model.
A lot of data, a lot of images, lots of articles into an endless stream of connections will often lead me into untravelled paths or keep pushing me into specific avenues, and lately, one of these has been the bone marrow. My immunology and molecular biology knowledge skyrocketed after the release of the mRNA vaccine, so ever since its announcement, I have been looking at one specific angle, arguably one of the most significant ones concerning the impact of SARS-CoV-2 and its Spike Protein on our bodies.
The bone marrow. Responsible for producing a myriad of our most important cells. The first breakthrough, to the detriment of the authors (who suffered significant pressure to retract their paper, and suffered academic damage), was evidence that the virus, and especially the Spike Protein damages HSPCs (stem cells) via pyroptosis (a form of cell death) via NLRP3 inflammasome (a potent inflammation-cascade initiatior).
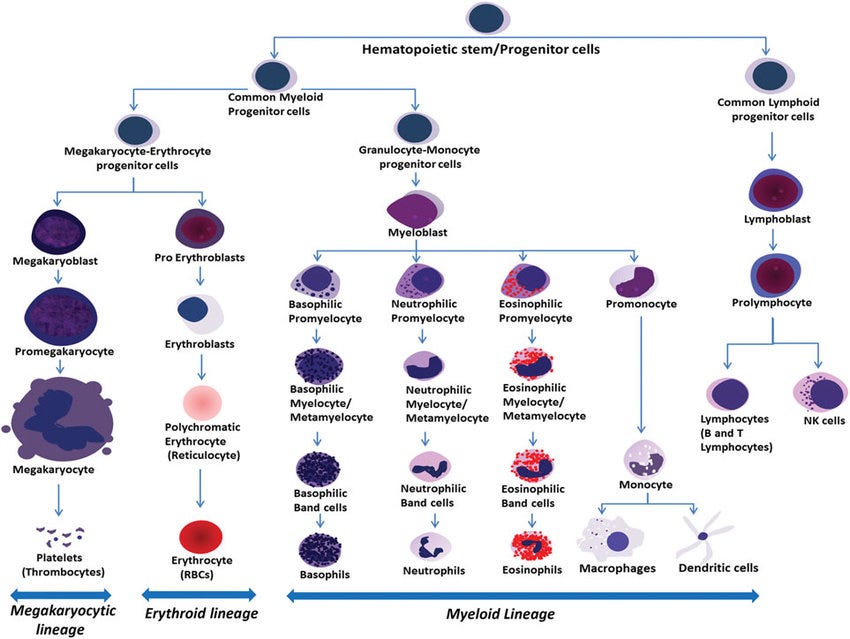
First, a brief explanation of what HSPCs are. These are multipotent cells, meaning they can differentiate into all types of blood cells, and this process is hierarchical, thus following an order. There are two main lineages in hematopoiesis.
Myeloid lineage (all the ones below)
Granulocytes → Neutrophils, eosinophils, basophils, involved in inflammatory responses, phagocytosis (eating invading pathogens), and allergic reactions
Monocytes, Macrophages - Antigen-presenting, phagocytic, tissue preparing and related to inflammation
Dendritic cells
Megakaryocytes → produce platelets
Erythrocytes → Red blood cells, carry oxygen, dependent on iron
Mast cells
Lymphoid Lineage
B cells, T cells, NK cells
Months later, another paper was published, making even more of a splash.
Per the title, infection, but at the time especially vaccination skewed stem cells, it damage the stem cells of gestating mothers, impacting their “fate”, which is a fancy way of stating they are damaged and there is less quantity and less types of cells because it affects the stem cells themselves. The impact of SARS-CoV-2 in the bone marrow is incredibly significant for the following reasons :
Direct and indirect disruption of the bone marrow leaves it susceptible for a “secondary hit”, and some pathogens can live in the bone marrow for long periods
A bone marrow reservoir (meaning the virus lives there quietly, evading the immune system) impacts your long-term immune system
If a pathogen kills a large portion of your immune cells, the bone marrow has to produce more, allied with systemic inflammation = inflammaging
One of my top concerns in regards to the long-term consequences of the pandemic has been “Inflammaging”, the accelerated aging of the immune system, easily observable in older folks, immune-compromised and immune suppressed people. Contrary to popular belief, exposure to chronic, low-grade inflammation, continued exposure to inflammatory responses (multiple infections), or systemic inflammation all contribute to “inflammaging”. I have been waiting for a long while now for someone to test the effects of Omicron (since, according to geniuses, it is just the flu, bro).
SARS-CoV-2 pseudovirus dysregulates hematopoiesis and induces inflammaging of hematopoietic stem and progenitor cells
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection primarily affects the respiratory system but may induce hematological alterations such as anemia, lymphopenia and thrombocytopenia. Previous studies have reported that SARS-CoV-2 efficiently infects hematopoietic stem and progenitor cells (HSPCs); however, the subsequent effects on hematopoiesis and immune reconstitution have not yet been described. Here we evaluated the pathological effects of infection of umbilical-cord-blood-derived HSPCs with the SARS-CoV-2 Omicron variant pseudovirus (PsV). Transcriptomic analysis of Omicron PsV-infected HSPCs revealed the upregulation of genes involved in inflammation, aging and the NLRP3 inflammasome, suggesting a potential trigger of inflammaging. Omicron PsV-infected HSPCs presented decreased numbers of multipotential progenitors (granulocyte‒erythrocyte‒macrophage‒megakaryocyte colony-forming units) ex vivo and repopulated primitive hematopoietic stem cells (Ki-67−hCD34+ cells) in an HSPC transplantation NOD-scid IL2rγnull mouse model (Omicron mouse). Furthermore, Omicron PsV infection induced myeloid-biased differentiation of HSPCs. Treatment with nanographene oxide, an antiviral agent, partially mitigated the myeloid bias and inflammaging phenotype both in vitro and in vivo. These findings provide insights into the abnormal hematopoietic and immune effects of SARS-CoV-2 infection and highlight potential therapeutic interventions.
In the introduction the authors raise the same point I had for a couple years now, the more pathogenic strains, and especially severe infections that often require hospitalization have a deep impact in the bone marrow, something also observed in a subset of Long Covid patients, but little is known on the effects of “systemic” infection, infection with a respiratory virus that avoids the lungs.
They decided to use Pseudovirus (PsV) model, which means certain cells expressing the Spike Protein, but incapable of replication, which “lowers” the safety necessary to conduct the research (Live virus needs strictly high biosafety, we can’t have another pandemic starting from a lab…lmao). They measure and show that HSPCs express the main entry receptors for the virus, ACE2, DPP4, and TMPRSS2, with ACE2 being abundant at both the gene and protein level.
They tested Wuhan, Delta, and Omicron PsVs by infecting HSPCs from umbilical cord blood (UCB) and found that all variants induced an inflammatory response, with an increase of IL-1β, IL-6, and TNF, and they chose to focus on Omicron due to obvious reasons. To understand what is going on with more detail, they did a transcriptomic analysis, analyzing which genes change when HSPC cells are exposed to Omicron PsV, this step usually gives any researcher deeper insight and significant “clues”.
Omicron PsV-infected HSPCs presented upregulated expression of genes related to myeloid differentiation (CD14, CSF1, and CD33) and inflammation (TNF, S100A8, NLRP,3 and IL-1β). Using an analysis tool for the most common (canonical) pathway,s they found upregulation of pathways related to senescence (zombie) cells, myeloid differentiation, and NLRP3 inflammasome.
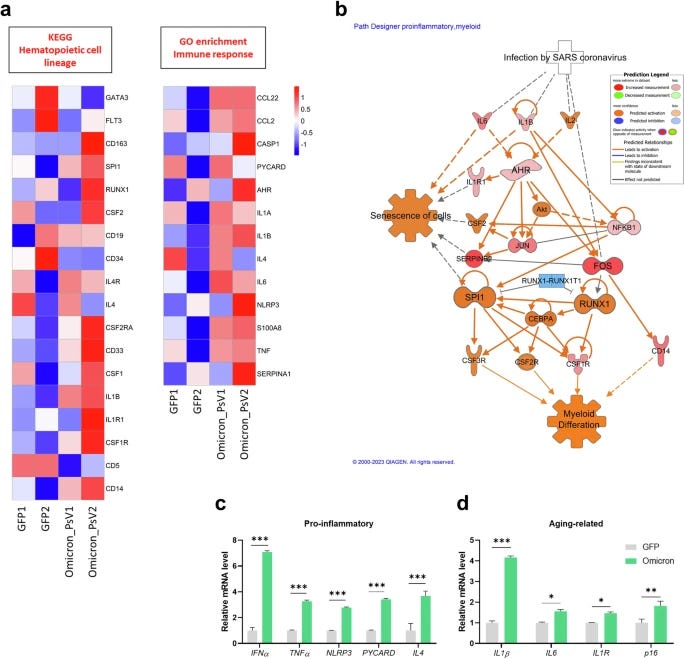
Expression of proinflammatory genes (IFNα, TNF, NLPR3, PYCARD and IL4), aging-related genes (IL1β, IL1R, IL6, and p16), and reactive oxygen species (ROS)-related genes (AHR, CYP1A1, AHRR, ID,O and NRF2) was increased in Omicron PsV-infected HSPCs. The expression of these genes is often related to “inflammaging”. The ROS-related genes, if you have been reading this Substack for the past 6 months, you should be intimate with all of these.
Their next step is testing how these changes affect hematopoietic cell lineage, meaning how these signals shift the production of cells in the bone marrow, if there is any significant change. Myeloid-related genes such as RUNX1, vWF, ITGB3, PU.1, and GM-CSF were significantly increased, while lymphoid-related genes were not altered upon Omicron PsV infection. To observe if this finding is valid at a cell level, they used a CFU assay (Colony Forming Unit), which measures the ability of progenitor cells to form different cell types.
The CFU assays showed that Omicron PsV infection resulted in a decrease in CFU-GEMM (multipotential progenitors), which are capable of differentiating into all types of blood cells, and BFU (erythroid progenitors), which give rise to red blood cells. Conversely, there was an increase in CFU-GM (granulocyte-macrophage progenitors), which are precursors to neutrophils and macrophages. This shift in progenitor populations strongly indicated a myeloid-biased hematopoiesis, skewing of blood cell production towards myeloid lineages at the expense of other lineages.
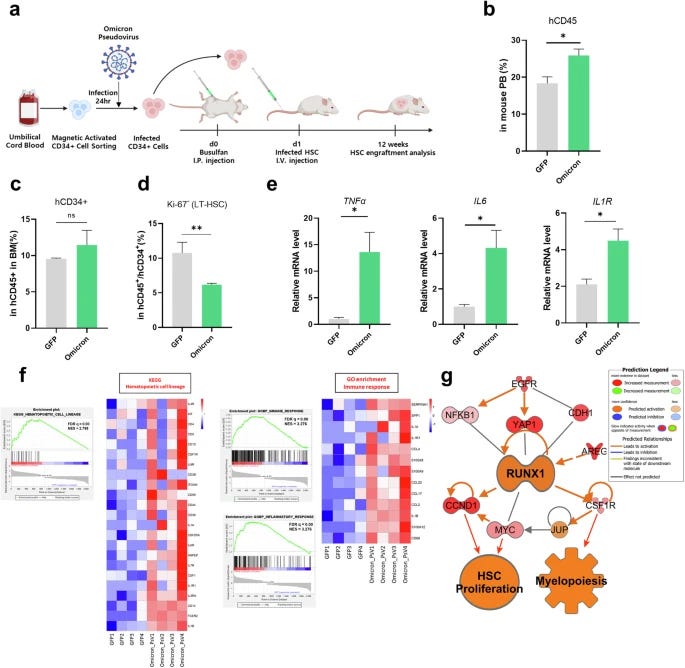
Their last step was to assess if these findings translate to in vivo, so they used a mouse model transplanting HSPCs infected with Omicron PsV, these mice are immunodeficient to allow the transplantation of these cells. After 12 weeks, they analyzed the bone marrow, peripheral blood, and spleen of these mice. The findings in vivo replicated the findings in vitro.
Expression of myeloid lineage-related genes (IL1R1, CSF1R, CSF1, CD36, and CD14) was significantly upregulated in Omicron mice, immune and inflammatory response alterations were identified via Gene Ontology enrichment analysis, and the expression of inflammation-related genes (CD68, IL1β, CCL2, S100A8, and SERPINA1) was also increased. Mice transplanted with Omicron-infected HSPC cells have a myeloid bias and an inflammaging phenotype.
This culminates into the finding that in the bone marrow of these mice, there is a reduction in Long-term HSCs, the most primite and self-renewing cells in the bone marrow, critical for long-term hematopoiesis, the inflammatory markers are typical inflammaging (replicating the one in vitro), and confirming the myeloid bias.
A myeloid bias means hematopoiesis is skewed towards the production of granulocytes, monocytes,and platelets at the cost of producing less lymphoid and sometimes erythroid (red blood) cells. This bias has inherent problems, and while context dependent, meaning the type of cells that are produced more as a compensatory response, it is often inflammatory, and contributes to long-term diseases such as cancer and neurodegeneration. Thus, we are thrown back to my January article.
AI-podcast of the article above.
While the mechanisms for months-long “hit” to the immune system are plentiful, from direct damage to the cells to abortive infection (the cell is infected, but the body kills the cell, the virus doesn’t replicate, but the cell dies), to specific parts of the Spike Protein “glueing” itself to specific receptors in the cells causing the above (the receptor used starts a chain-reaction that kills the cell) would explain a severe primary hit, and a delay recovery, but not a lasting, sometimes 10 months long effect.
What would explain is the virus directly affecting the bone marrow, be it my systemic inflammation, something that will become a trend with Omicron and its lasting effects in the body, or directly impacting or infecting bone marrow cells. There is no other more pernicious player in the bone marrow than HMGB1.
The One (protein) to rule them all. (one of my most significant articles to date)
The impact of HMGB1 on an endless list of diseases by itself is large enough that I could dedicate multiple extensive articles covering every angle,, but here we look through a specific perspective, HMGB1 known as “Endotoxin-binding Protein”, SARS-CoV-2, and its Spike Protein, also widely recognized as being able to bind to Endotoxin.
This interaction forms a protein complex consisting of the 3 proteins, and in the case of secondary infection, gut dysbiosis (directly caused by Omicron at a significant population level) and also by vaccine-induced inflammation (given that the LNP and the vaccine-Spike will pierce through everything, also being contaminated by enough Endotoxins on its own).
This protein complex will enhance TLR4 and RAGE signaling, one of the most pernicious feedback loops in inflammatory diseases and infection (sepsis especially) this initiates an exacerbated NLRP3 inflammasome activation and pyroptosis, inducing HSPC damage. This also creates a lasting enough loop to induce inflammaging. This will inevitably create an immune suppressive state biased towards myeloid cells.
If you recall the main paper of this article, you will find a curious number of proteins being produced in HSPC cells after being infected by Omicron PsV. AhR, IDO, IL-6, and this means one thing. Interferon-gamma (IFN-y) is produced significantly enough at a point, as the evidence demonstrates IFN-y promotes myeloid-biased differentiation. IFN-y and HMGB1 form an axis that distupts the bone marrow, creating a feedback loop by upregulating specific inflammatory signaling leading to chronic inflammation and myelosuppression.
This creates a “hit and run” effect, where SARS-CoV-2 (the hit) leaves the door open for a secondary infection (viral, fungal or bacterial, adding complexity to all of this) or predisposition for immune reactions (the run), leading to a transient immune dysregulation, including accelerated aging of the immune system, lymphocytes in particular in certain instances (mRNA vaccines).
Each subsequent infection acts as an additional inflammatory challenge to the already stressed hematopoietic system, and this repeated inflammatory stress is one of the key mediators of HSPCs aging and myeosuppression. This will occur on long-enough timelines so nobody who isn’t looking at the entire picture will miss it. How it goes, as I previously wrote crudely, now within this context.
SARS-CoV-2 Infection →Myelosuppression → Increased Infection Risk → Worsening Myelosuppression → Negative feedback loop → repeat step one
SARS-CoV-2 infection -> Initial myelosuppression and immune dysregulation.
Increased susceptibility to secondary infections.
Secondary infections -> Further inflammatory stress on bone marrow -> Worsening myelosuppression.
This cycle can perpetuate and lead to more severe and prolonged myelosuppression over time.
While I may repeat this next passage in the Cognitive Strike Part III, a skewed ratio between other cells and lymphocytes is easily observable in Alzheimer’s Disease, neutrophils in the aforementioned study and in this paper too. Another study.
“Changes in peripheral blood cell profiles, particularly involving leukocyte, lymphocyte, neutrophil, and CD8+ T cell counts, as well as the NLR and the CD4/CD8 ratio, are closely associated with AD.”
A recent peer-reviewed study, which I will cover separately, recently proposed and demonstrated the central role of Epstein-Barr Virus (a herpes virus) in Multisystem Inflammatory Syndrome in Children. Overlooked here remains the role of Herpesvirus, the immunological changes brought by SARS-CoV-2 leading to reactivation of these viruses and their role in myelosuppression and the bone marrow. To the surprise of no one reading me for any length of time, Herpes infections have skyrocketed since 2020.
Covid was primarily a “Neurostrike weapon”, everything else secondary, but I would argue the targeting of the bone marrow via multiple mechanisms was elegantly designed by some PLA scientist. My suggestion on how to deal with both the short and long-term consequences remains the same, just limit the ever-living hell of inflammation for 4 to 8 weeks. I will inevitably write a “Part II” at some point.
Consider becoming a supporter, and if you are one, thank you !








I wish everyone a great weekend ahead.
Undecided if Cognitive Strike Part III should be a big one, or break down in fewer papers. Something else coming in the meantime if I feel like it.
6th Gen Warfare =).
A word of warning to anybody who is trying to limit inflammation though is to time thr intervention carefully. Since Omicron the bioweapon has a wonderful way of suppressing the initial immune response, particularly inflammation. So the first few days should be spent making sure you get a reaction going , spray interferon directly if you can get it (and doubling down on things with antiviral property like IVM, Berberine, Zinc, Quercetin). Then from about day 4/5 it’s time to hit the inflammation. The timing is tough and things are made doubly hard by this initial suppression followed by widespread inflammation, coagulation and oxidative stress and damage.