The Long-term consequences of SARS-CoV-2 infection - Part II
Upon this immune system, I shall build my plague
The article was a little delayed because the Supplementary Data from 2 of the papers here are a gold mine of information, and data points, although highly complex. There is so much gold there though =).
In July I wrote an article talking about the consequences of SARS-CoV-2 infection, covering, mostly, the Wuhan strain, which is the most severe one, and the paper covered, and even “mild to moderate” infection-induced lasting immunological changes. In the article, I argued it was because of the high expression of HMGB1, Wuhan directly affecting the bone marrow, and potentially its superantigenic aspect.
AI-generated podcast, does a good job of covering the paper and my article with the significant points mentioned below.
This is not exactly a follow-up, but building up on the article, because we now have multiple studies
on the long-term effects of “mild infection/Omicron bro” on your entire physiology. In simple terms, back in the nasty strain days, even a mild infection caused significant immunological changes, that not only lasted but progressed for months, up to 10 months.
Baby T-Cells (Recent Thymic), T-Cells, CD4, CD8, a specialized niche of B1-like cells, and lastly, but not least a shift in proteins from a Th1 (inflammatory) to Th2 (some of the proteins are anti-inflammatory, but often play a role in allergic reactions). Among the many causes, the reader will be aware of the ones I proposed. The Spike Protein interactions with bacterial toxins have now proved superantigenic profile, reactivation of latent viruses, and endogenous retroviruses. In the end, I argue, that the virus and Spike Protein regardless of source, can suppress the bone marrow for a while.
A clarification before we delve into the first paper. The reason I focus on Long Covid is not out of the sheer complexity, one of the hardest puzzles to be solved, but if you know how to look at the data, with the right analytical framework, and a few unconventional methods you can use Long Covid as a forecasting tool, reliable one.
One of my most recent articles covered how even a mild Omicron infection increases cardiac damage markers, so we will remain in line with this subject for the first paper.
Post-acute sequelae of SARS-CoV-2 cardiovascular symptoms are associated with trace-level cytokines that affect cardiomyocyte function
PASC is now an umbrella term, which I don’t completely disagree with, but it makes a distinction between subgroups harder, especially based solely on symptoms and not biomarkers, and there is a significant variation between each group and their markers. There is also long-lasting dysfunction in PASC individuals, often overlooked. The most common manifestation of PASC is cardiovascular, but we lack insights into the long-term (higher than 12 months) inflammation and dysfunction.
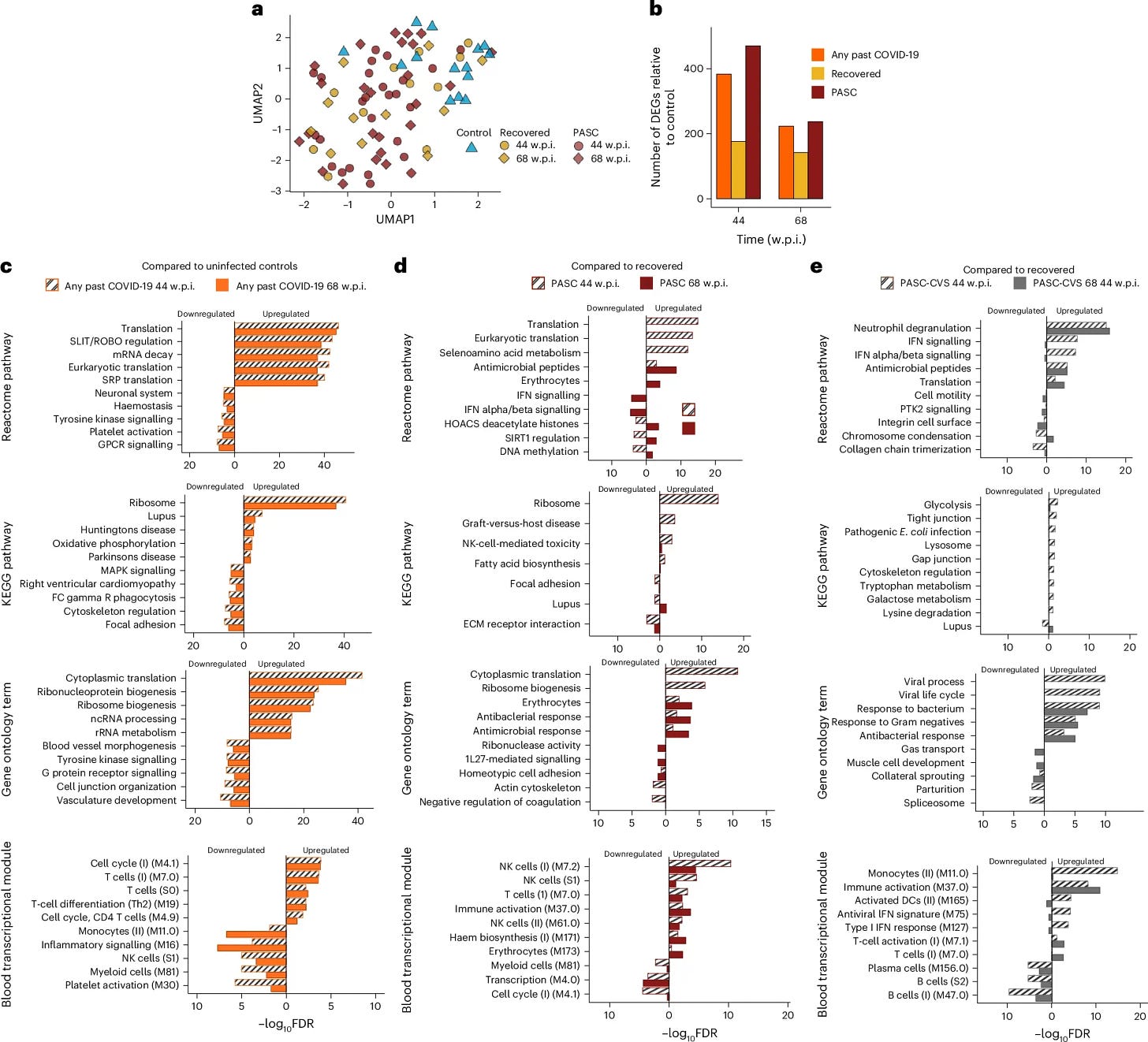
Their first test was a transcriptomic analysis of blood samples which revealed distinct differences in gene expression profiles between individuals with PASC-CVS and those who had recovered from COVID-19. RNA sequencing (RNA-seq) of whole blood demonstrated persistent activation of immune pathways in PASC-CVS individuals up to 18 months post-infection.
Differentially expressed genes (DEGs) were significantly enriched in pathways associated with neutrophil degranulation, interferon signaling, and platelet activation. These findings suggest ongoing immune dysregulation in PASC-CVS patients. Cytokine profiling using highly sensitive nanotechnology-based immunostorm chips detected elevated levels of Interleukin-1β (IL-1β), Interleukin-6 (IL-6), Interleukin-12 (IL-12), and Monocyte Chemoattractant Protein-1 (MCP-1).
These proteins were found at femtogram levels, significantly lower than the detection thresholds of conventional assays, so the authors used a novel nanomeasurment method, implicating their participation in the dysfunction. These proteins are known mediators of inflammation and immune cell recruitment, indicating a possible mechanism for sustained cardiovascular symptoms.
It is also worth noting that there is a significant upregulation of bacterial responses, at 68 weeks post-infection PASC show higher production of antimicrobial peptides, pathways responsible for fighting bacteria, T, and Natural Killer cell activity. Cardiovascular ones show the same plus enhanced neutrophil degranulation (important to fight bacteria), even stronger bacterial response, and higher monocyte, and dendritic cell activity.
Why does this is significant ? Because it implicates multiple mechanisms. First, one of the hallmarks of all most forms of PASC is the amyloidal burden, and amyloid proteins are used by the body as a last resort to deal with bacterial infections and bacterial components. This persistent heightened immune response could be due to viral persistence, dysbiosis, and the body mishandling persistent toxin exposure.
Given the amount of cytokines is minimal, “trace levels” the authors decided to use human-induced Pluripotent Stem Cell-derived Cardiomyocytes (hiPSC-CMs), these are cells that can be used to model cardiac diseases, especially in relation to cardiomyocytes. They cultured these cells with a mixture of the cytokines mimicking the levels found in the PASC-CVS plasma.
The results revealed significant functional impairments in the cardiomyocytes treated with PASC-CVS cytokine mimics compared to those treated with controls or recovered plasma mimics. Specifically, there was a marked reduction in contraction amplitude, a critical measure of cardiomyocyte pump function, and slower upstroke velocities, indicative of impaired electrical conduction.
Enhanced relaxation velocities were also observed, pointing to disruptions in the excitation-contraction coupling of cardiomyocytes. These changes were synergistic, as individual cytokines did not produce similar effects.
Proteomic analysis of plasma samples demonstrated further molecular alterations in PASC-CVS. Among the 569 proteins identified, the involved in coagulation and complement activation were significantly upregulated in PASC-CVS plasma. Notably, proteins like prothrombin, complement factor B, and complement C1r were enriched, supporting the hypothesis of a thromboinflammatory phenotype.
The presence of serum amyloid A-4 protein, associated with enhanced clotting and venous thromboembolism risk, aligns with the micro clotting patterns observed. These findings indicated a persistent continuous dysregulation of coagulation and the complement pathway (which participates in the former) and ongoing inflammatory and tissue damage.
Chronic exposure to the cytokines mentioned here can alter calcium influx in cardiomyocytes (and in other cells too), reduce contractility, and compromise cardiac output, the persistent complement activity and amyloid present will cause more tissue damage thus creating a feed loop that produces microclots, inducing the long-term cardiovascular symptoms.
“Ok, but bla bla bla Long Covid bla bla bla”. That is why the next paper has been among one of my favorites in recent memory because now you will argue against millions of data points from hundreds of thousands of people, and there is vaccinated vs unvaccinated differentiation.
Trends of common laboratory biomarkers after SARS-CoV-2 infection
Results
The general cohort included 361,061 matched pairs, with 26M laboratory results. The effects on most biomarkers lasted 1–4 months and were consistent with anticipated changes after acute viral infections. Some biomarkers presented prolonged effects, consistent across the general and lab-specific cohorts. One group of such findings included a 7–8 month decrease in WBC counts, mainly driven by decreased counts of neutrophils, monocytes, and basophils. Potassium levels were decreased for 3–5 months. Vaccinated individuals’ data suggested potentially smaller effects on WBCs, but cohort sizes limited this analysis.
Conclusions
This study explores SARS-CoV-2 infection effects on common laboratory biomarkers, characterizing the direction and duration of these effects on the largest infected cohort to date. The effects of most biomarkers resolve in the first months following infection. The most notable longer-lasting effects involved the immune system. Further research is required to characterize the magnitude of these effects among specific individuals.
Another “gold mine” for data in a sense. Here there is a stratification between unvaccinated, and vaccinated, among other variables. Many of the findings in this study reflect prior clinical, published observations of the significant effects SARS-CoV-2 infection has on the body. But instead of separated findings, presented here is an encompassing picture of the effects, and with special attention to the long-term effects.
I spent the last 40-something hours making absolutely sure I got the data analysis right, using multiple different methods, not only for accuracy. And the data here is absolutely clear. The unvaccinated (UV) suffer a significant, profound, and larger initial impact, while the vaccinated (V) don’t experience such, yet… The unvaccinated recover much faster, perhaps a compensatory response, while the vaccinated suffer more fluctuations and a delayed impact.
Red Blood Cells and Hemoglobin, necessary to carry oxygen, and their need for iron being crucial suffer a significant impact on both groups, but UV recovers faster and more consistently over time in comparison to the V group, which has a slower recovery, less consistent, with a trend to secondary dips in later time points.
The groups have some intriguing differences when it comes to immune cells. Lymphocytes suffer a significant impact, which tends to recover but remain impacted even after months in both groups. Neutrophils show a small but significant negative impact, while in the V group, they suffer a positive impact (perhaps a compensatory response only present in the vaccinated ?), it dips and recovers later on in this group.
In the UV group for most parameters (LYMP.abs, LYM%, NEUT.abs, NEUT%, MONO.abs, MONO%, EOS.abs, EOS%, BASO.abs, BASO%), the initial negative impact lessens over time, showing a recovery trend. LYMP.abs and LYM%, experience a pronounced negative impact later, with lagged recovery that dips. MONO.abs also experience a negative impact later. These findings are in line with, among other things, the susceptibility of anyone infected with SARS-CoV-2 to experiencing bacterial infections after (a trend common in the V group).
There is a significant impact on potassium in both groups that lasts around 5 months. A similar trend is found in biochemical markers. CREATININE, CALCIUM, PHOSPHORUS, PROTEIN-TOTAL, ALBUMIN, CHOLESTEROL, CHOLESTEROL-HDL, CHOLESTEROL-LDL calc, ALK. PHOSPHATASE, AST (GOT), ALT (GPT), GGT, LDH, TRANSFERRIN.
The UV group suffers a significantly negative impact, with a trend towards recovery later on with some markers fluctuating, but recovery trends to baseline, although Urea and CPK experienced delayed negative effects. While the V group suffers a less initial impact, with a less consistent recovery and later worsening in some of these markers, with special attention to liver enzymes.
UV suffers a delayed renal burden, while the V, among other burdens, the liver, in line with glucose fluctuations experienced in vaccinated people 12 months later after an infection. The data is clear, UV exhibits a pattern of a significant initial impact but with a faster and more consistent recovery, while the V group suffers a less significant initial impact, but suffers from a more persistent fluctuation and delayed negative impact, pointing towards slower, inconsistent recovery.
And lastly but not least, a study from China, a country without mRNA vaccines. The full paper was provided by a follower, thank you =D.
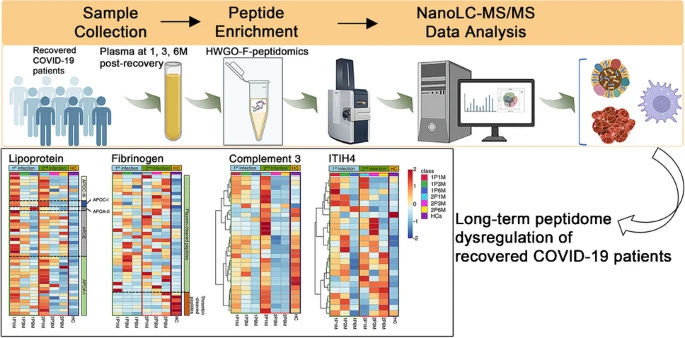
Long‑term dysregulation of plasma peptidome in mild and multiple COVID‑19 recovered patients revealed by a novel efcient peptidomics workfow
After recovering from COVID-19, many patients experience “long COVID” symptoms. Existing research has predominantly focused on moderate to severe cases, with limited studies examining mild cases and recurrent infections. The circulating low-molecular-weight (LMW) peptidome, involving lipid metabolism, coagulation, and immune pathways, is crucial for understanding COVID-19’s long-term effects. We developed a peptidomics workflow utilizing solid-phase extraction with highly wrinkled GO-Fe3O4 composite materials (HWGO-F) and nanoLC-MS/MS detection. By altering the pH, HWGO-F enhances plasma peptide adsorption and purification. Compared to traditional methods, our workflow offers improved detection depth and reproducibility for over 70% of peptide signals with CV < 20%. We investigated plasma peptide profiles in mild COVID-19 patients post-recovery from single or second infections. The findings indicate persistent abnormalities in initial COVID-19 infections’ plasma peptide profiles, gradually diminishing over time. Secondary infections prolong recovery. Disrupted functions include lipid metabolism, coagulation and complement cascades, and infection-related pathways. Lipid metabolism may normalize within 3 months, while coagulation and immune abnormalities can last 3–6 months. After secondary infections, lipid metabolism irregularities may last at least 1 month, with extended coagulation and immune imbalances. These results provide a theoretical foundation for understanding the widespread occurrence of long COVID and guide recovery care for mild cases.
One thing I admire and personally love is when researchers come up and design new, better, more accurate detection methods to achieve deeper insights, similar to the first Long Covid paper where the authors used a way to measure proteins at nano levels, the authors here follow the long-term peptide dysregulation.
The long-term consequences of mild infections remain understudied, and this studied aims to bridge the gap in understanding, and finding the correlation between persistent symptoms after mild infection and functional changes in the body. There is a disruption in lipid metabolism, and proteins such as APOA-I, APOE, and APOC-III suffer significant changes.
Lipoproteins such as these play a critical role in transporting cholesterol, vitamin absorption, and immune modulation (among many other effects, such as avoiding misfolded protein build-up and accumulation). Their degradation, which is probably driven by MMPs (Matrix Metalloproteinases, found elevated here) causes an increase in these. They normalize after 3 to 6 months but tend to take longer if there is another infection within these 3 months.
Consistently with past and recent evidence, and consequentially many of my recent articles,s the authors found persistent coagulation cascade dysfunction even in mild infection. Thrombin, an enzyme essential for converting fibrinogen into fibrin during clot formation was suppressed up to six months post-infection. Plasmin, responsible to break down fibrin clots remained elevated.
Fibrinogen cleavage products, such as fibrinopeptide A (FpA) were observed to remain dysregulation, thus demonstrating that the recovery in the coagulatory system is slow, pro-thrombotic (clot forming), and hyberfribrinolytic (producing too much fibrin). The complement system which is critical for fighting pathogens, coagulation, immune responses, and regulating inflammation was also found to be dysfunction.
Complement factor I, which cleaves C3 into inactive fragments, was shown to produce altered levels of complement component 3 fragment f (C3f), a peptide with inflammatory and insulin-like growth factor-enhancing properties. While C3f levels declined during recovery, they remained abnormally low compared to healthy controls, especially after secondary infections. This indicates continuous inflammation, and delayed tissue repair, among other effects.
This can be seen by measuring and tracking Acute-phase proteins, proteins produced in response to inflammation. Inter-Alpha-Trypsin Inhibitor Heavy Chain H4 (ITIH4), SERPINA1 (alpha-1-antitrypsin), and Haptoglobin (HP) were found to exhibit persistently elevated peptide levels during the early months of recovery. ITIH4 is involved in modulating the inflammatory response to tissue trauma, while SERPINA1 and HP are key players in countering protease activity and oxidative stress.
The sustained elevation of these markers reflects ongoing low-grade inflammation. SERPINA1 should be taken note of because it will be important this year. It is also interesting to note that this paper takes into consideration reinfection and how this affects recovery, often adding one to two months to recovery time, and increasing the burden.
A direct comparison between the 3 papers is difficult unless it is done with a distinct analytical framework, but if you take into account many of my articles in the past 9 months, a rather peculiar picture can be seen. There is no such thing as a “mild, just the flu bro” infection. As I have stated in 2021, 2022, and so forth. The trick is supplementing and aiding the body to recover before the inevitable next hit, because bone marrow suppresion is happening.
Throughout this entire year, a lot of unforeseen, odd immunological and health events will take place, and yet few will make sense or accept reality. Not my place to, anyway.
Much shorter, more to-the-point articles to come.
I am grateful for your support, because it enables my unorthodox analysis =), and other writings.





In the past few months, there have been now almost a dozen papers, covering different organs on changes in microstructure, microvasculature, and significant changes in coagulation, meaning microclots.
A few, shorter, direct-to-the-point, articles on that tomorrow, adding to it. One is not so direct, because it is HIGHLY impact.
Any mistake here, I will correct it tomorrow. Cognitively spent.
I wish you all a great week ahead. Its winter in the Northern Hemisphere, significant outbreaks of respiratory disease, be sure to take some extra Vitamin D, Selenium, Magnesium, perhaps Potassium per the article.
An absolute cracker of an article. Especially bringing in the V v UV comparison. There is some hope in here that it does seem most can recover if they look after their bodies properly. But it needs more than just supplementing. Sleep, fasting, exercise, diet etc, all combined. Basically everything you have been saying for 3+ years is now becoming glaringly the best way to survive the onslaught and hopefully recover.
Would love to hear more about why SERPINA1 is going to be so important this year.