The quest to understand the hidden trends and outlier effects of SARS-CoV-2 and its systemic onslaught has led me to an extraordinary interdisciplinary journey. Because I was very aware early on (mid-2020) misfolded proteins would play a substantial, significant role in the long-term consequences of widespread infection with a bioengineered chimera.
Thus with extensive research, you can infer outcomes specialization often misses. I have long stated that misfolded proteins are highly complex, serve more than one purpose, and are most likely a forgotten defense mechanism (preserving long-term memories at the cost of new ones, extremely useful in a hunter-gatherer society), Herpes play a role, these proteins, not even prions “are forever”.
The amount of crap pushed by so many “influencers” was so high it led me to write the following article (as usual, AI podcast below).
During my exploration of the Glymphatic System, I came to understand the main variables causing misfolded protein accumulation:
Failure of the antioxidant system
Impairment of the circulatory system, especially the microcirculatory system (capillaries)
Last, clearance rate. Failure on how fast the body can clean, rather than how much it builds up
I have argued that even the highly infamous and widely feared Creutzfeldt-Jakob Disease (CJD), the poster child for prion disease was more complex than assumed and bound to the laws of biophysics. Alas…
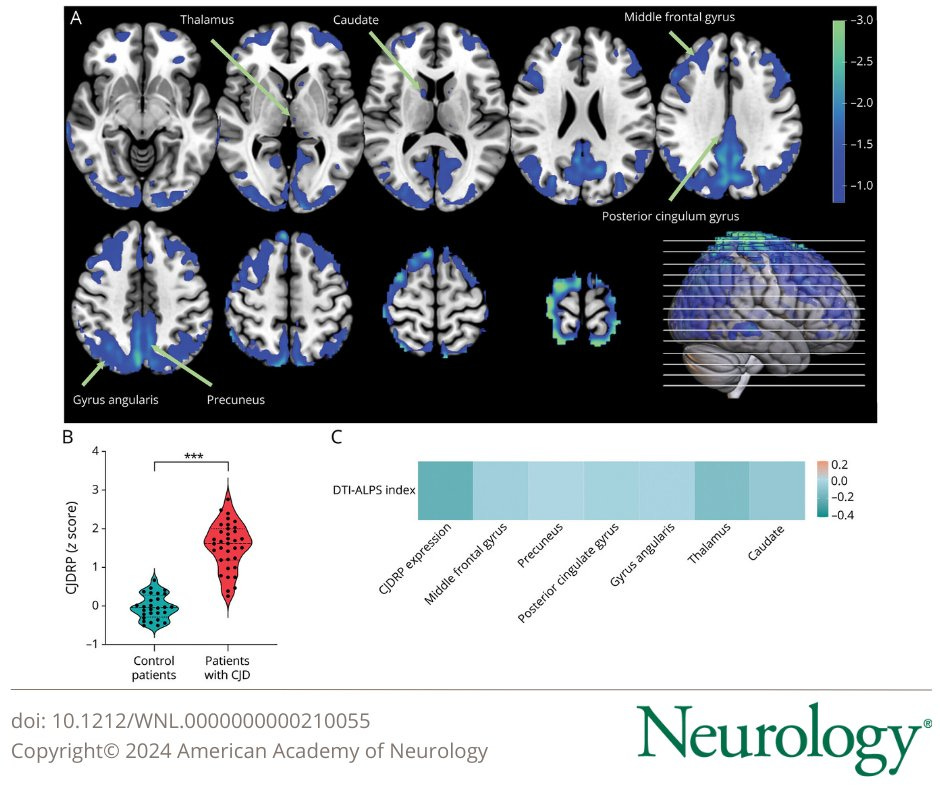
In this study, researchers compared 35 CJD patients, 25 having sporadic, meaning “unknown cause” and 10 carrying a specific gene with 28 controls. Symptomatic patients had significantly lower DIT-ALPS. This is an MRI-based test to measure water diffusion along perivascular spaces, exactly where the glymphatic system lies.
Among the patients carrying the G114V (a mutation in the prion protein gene that causes a change in that specific amino acid, thus making the good prion, into a bad prion, in a sense) only one carrier experienced a significant glymphatic decrease, losing 8.2% of its clearance rate in 3.3 years, 2 standard of deviantions greater than other carriers. One other carrier was a significant decrease in brain metabolism in different regions but remained asymptomatic.
This indicates that rapid changes in glymphatic function are more predictive of disease onset and symptom development than metabolic measurement, at least in some patients. Interestingly hypometabolism affects different parts of the brain, but glymphatic dysfunction reflects a systemic clearance failure, so glymphatic dysfunction can serve as an independent and earlier marker (there is evidence hypometabolism is not the most reliable marker).
Source for the paper below, sadly paywalled.
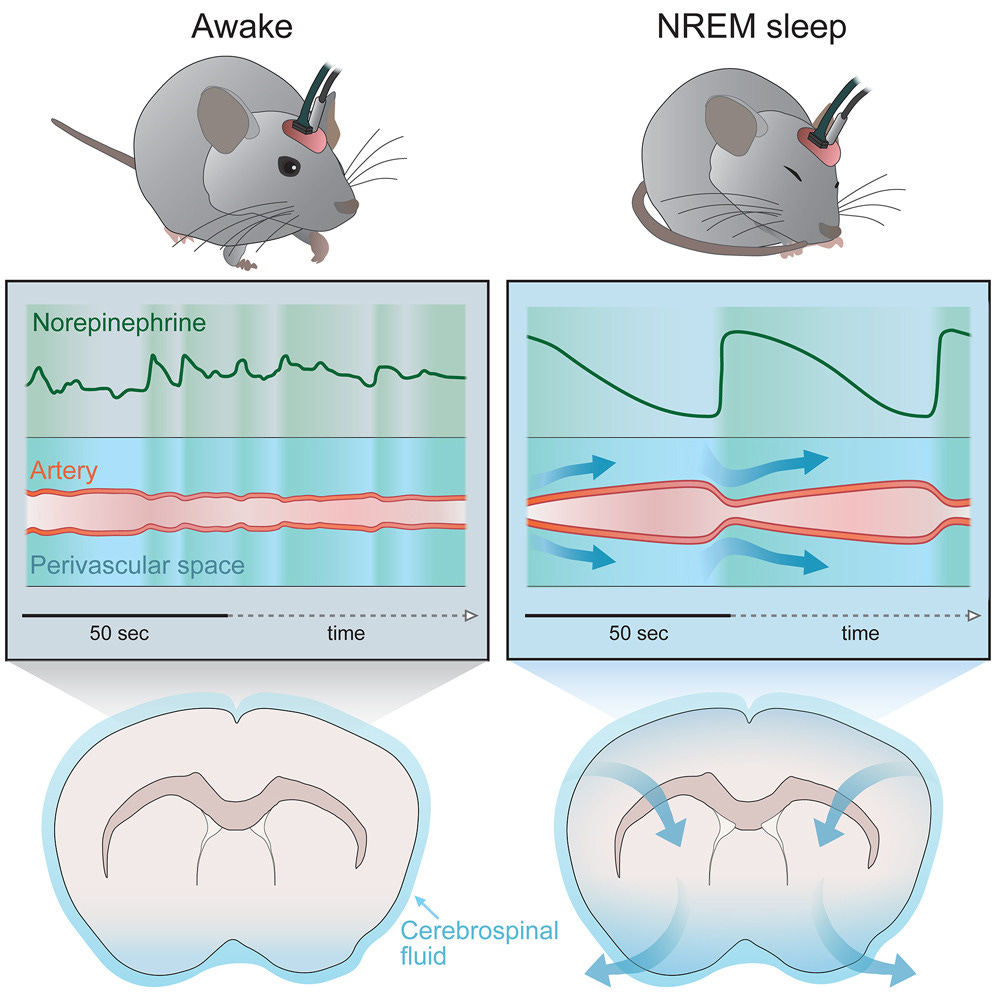
That the Glymphatic system is essential for the clearance of waste in the brain we are very aware already, but how it happens while we sleep ? The actual biophysical mechanism. And in the paper above that is exactly what the authors uncovered.
While you sleep, the brainstem releases tiny amounts of norepinephrine about every 50 seconds, which thus makes the microvasculature and microvasculature contract in a pulsating manner, creating a flow, that affects the perivascular space (where the glymphatic system is).
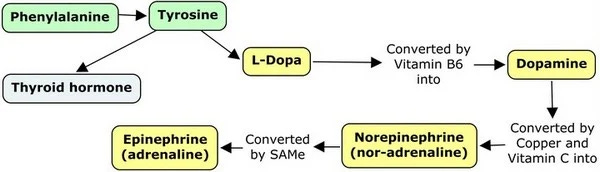
In case you forgot Norepinephrine (also known as noradrenaline) is a major neurotransmitter that also acts as a hormone, it is responsible for transmitting signals between neurons and other cells, but its hormonal role is controlling blood pressure. It is also part of a pathway that controls reward signals in the brain, learning, and memory. Norep belongs to the Dopamine Pathway which can be seen below and it will be important throughout the rest of the year.
I talked recently about Norep, in the article covering the microvascular impact of SARS-CoV-2 in which the authors found that exposure to the S1 part of the Spike Protein causes microvascular dysfunction and the microvasculature is less responsive to Norep. This pathway will increase in importance as the years go by.
Another interesting part of the paper, and given the global abuse of sleeping aid drugs, the authors decided to test what effects drug-induced sleep had. They used zolpidem, and in zolpidem-treated mice, norepinephrine waves during deep sleep fell 50%, and the clearance rate FELL more than 30%. Melatonin is a far superior option to drugs, with significant benefits, and plays an important role in this process.
Another one of my arguments presented in late 2023, referred to in the introduction, is the particularly under-researcher and hidden role Herpes viruses play in neurodegeneration, especially in long-term consequences. And misfolded proteins are often an unrecognized protective mechanism, the body's last resort.
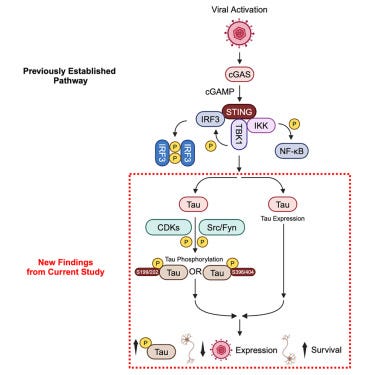
Anti-herpetic tau preserves neurons via the cGAS-STING-TBK1 pathway in Alzheimer’s disease
• HSV-1 protein expression increases with Alzheimer’s disease progression
• In Alzheimer’s disease, phosphorylated tau colocalizes with HSV-1 protein
• Following HSV-1 infection, tau is phosphorylated downstream to cGAS-STING pathway
• Post-infection tau phosphorylation reduces viral protein and boosts cell viability
The direct role of HSV-1 (Herpes Simplex Virus-1) in the development of Alzheimer ’s-like diseases has been long proposed, but so far it lacked direct evidence, although for years the expression of HSV-1 protein during Alzheimer’s Disease (AD) during all stages of the disease has been observed, so far it was very hard to obtain good imaging given the limitations of staining techniques directly.
Thus the researchers used a novel technique called Decrowding Expansion Pathology (dExPath), which enables manipulation of the tissue “inflating” brain samples, allowing for remarkable nanoscale imaging. Using this technique they found HSV-1 proteins ICP27, ICP4, gB, and VP16 were found in all brain samples regardless of disease severity, but ICP27 increased with diseased severity in specific brain regions studied here.
They found that both neurons and microglia had ICP27, neurons are the “natural targets” of the virus, and microglia “eat” infected neurons. In this study, the authors didn’t find colocalization of ICP27 and Amyloid Beta (the bad one), a contract tau. The authors propose that Amyloid beta is most likely an anti-bacterial and anti-fungal response and perhaps Amyloid Beta mediates a response to HSV-1, while Tau responds inside the cell.
Phosphorylated Tau (pTau - when “good tau” becomes “bad” tau) was found to reduce ICP27, suggesting an anti-herpetic function. This occurs by tau translocating to the nucleus, impacting RNA transcription and assembly, thus inside the cell tau may play a role in inhibiting Herpesvirus replication. pTau here is shown to increase neuron viability, which benefits both host and virus alike.
Where the damage comes from is “chronic phosphorylation”, as seen in Alzheimer’s which leads to accumulation. Short-term tau phosphorylation may act as a beneficial antiviral response, whereas chronic will lead to toxicity, and if will be caused by repeated HSV-1 reactivation, which happens by aging, stress, and immune depression/suppression. Or…
Repetitive injury induces phenotypes associated with Alzheimer’s disease by reactivating HSV-1 in a human brain tissue model
Infection with herpes simplex virus type 1 (HSV-1) in the brains of APOE4 carriers increases the risk of Alzheimer’s disease (AD). We previously found that latent HSV-1 in a three-dimensional in vitro model of APOE4-heterozygous human brain tissue was reactivated in response to neuroinflammation caused by exposure to other pathogens. Because traumatic brain injury also causes neuroinflammation, we surmised that brain injury might similarly reactivate latent HSV-1. Here, we examined the effects of one or more controlled blows to our human brain model in the absence or presence of latent HSV-1 infection. After repeated, mild controlled blows, latently infected tissues showed reactivation of HSV-1; the production and accumulation of β amyloid and phosphorylated tau (which promotes synaptic dysfunction and neurodegeneration); and activated gliosis, which is associated with destructive neuroinflammation. These effects are collectively associated with AD, dementia, and chronic traumatic encephalopathy (CTE) and were increased with additional injury but were absent in mock-infected tissue. Blocking the cytokine IL-1β prevented the induction of amyloid and gliosis in latently infected monolayer cultures after scratch wounding. We thus propose that after repeated mechanical injuries to the brain, such as from direct blows to the head or jarring motions of the head, the resulting reactivation of HSV-1 in the brain may contribute to the development of AD and related diseases in some individuals.
That doesn’t bode well for me… lol. Repetitive head injury or jarring (fast, chaotic) motion with the head can reactivate HSV-1, thus leading to neurodegeneration.
My brain works somewhat as the organic version of an advanced Machine Learning algorithm, and a fairly complex Language Model, which means I sometimes suffer from the same shortcomings as both and will miss the obvious right in front of me. It only dawned on me to see if there is any research on SARS-CoV-2's impact on the glymphatic system. There is an extensive, large body of research on SARS-CoV-2's impact on the pathways and proteins discussed in this article.
Glymphatic system dysfunction in recovered patients with mild COVID-19: A DTI-ALPS study
As the first study to evaluate glymphatic system function in patients with COVID-19, our result is consistent with the atrophy of brain structure10,32 and particularly the disturbance of cerebrospinal fluid circulation
In conclusion, we found that, with or without sequelae, the DTI-ALPS index is lower in recovered COVID-19 patients within four months, which might reflect impairment of glymphatic function. The decline in glymphatic function is more pronounced when the infected person is older.
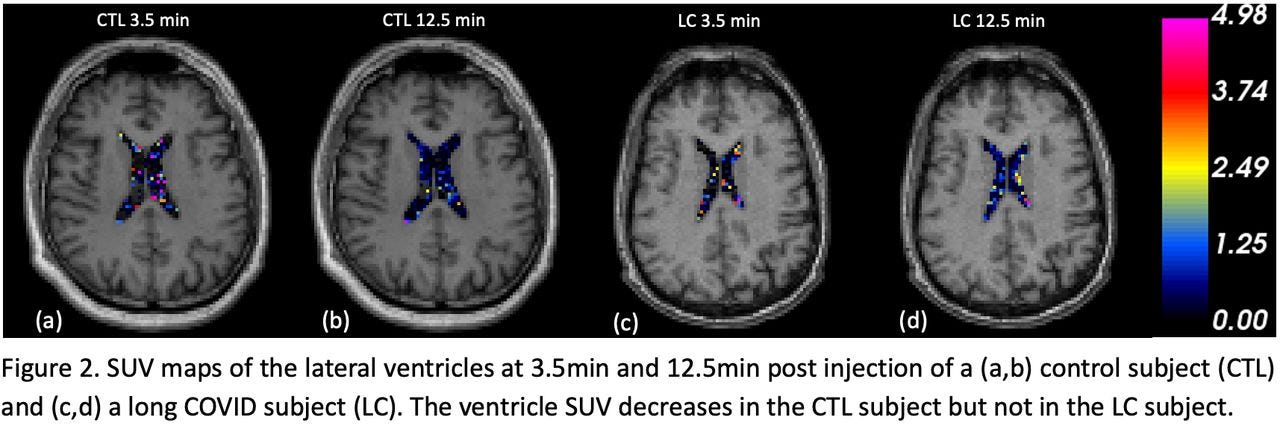
Impaired Cerebrospinal Fluid 18F-FEPPA Clearance in Long COVID Suggests Altered Glymphatic System Function
Our study demonstrates reduced lateral ventricle 18F-FEPPA CSF clearance in long COVID patients. This preliminary result provides potential evidence of glymphatic system dysfunction associated with neurological long COVID and may imply tauopathy in patients.
How do you address these changes ? It is not necessarily hard. Maintaining proper Vitamin D levels should be the primary goal, followed by appropriate levels of magnesium and selenium (which we are all, somewhat deficient), some may need Iron, B complex, but the most important is antioxidation support. Glymphatic system failure is often preceded, by or a direct byproduct of NRF2, the master antioxidant of the body.
Melatonin, NAC+Glycine, Curcumin, Resveratrol, and omega-3 are great for glymphatic support among many other positive effects in the body. These will also keep Herpes reactivation in check, avoiding it, rather than merely “fighting” it. One of my favorites, Gingko Biloba is excellent for the entire circulatory system =), so does Vitamin C.
The next article will be Cognitive Strike 2, the first covered how Covid was accelerating neurodegeneration, Part II will cover how extensive, some of the deeper mechanisms and especially which part exactly and function it is affecting. I do wonder how many of you are forgetting words with higher frequency, decaying spatial awareness, but especially not good with math and complex tasks anymore…
If you support my work, or decide to start supporting it, thank you =), it enables me to research !







"I do wonder how many of you are forgetting words with higher frequency, decaying spatial awareness, but especially not good with math and complex tasks anymore"
Can confirm all of the above...as well as general sluggishness.
Ginko is used in Germany for stroke which made me recall something else used for stroke... DMSO.... then there's the spicy foods, cayenne, alliums.. and rosemary. Cleavers the herb, is supposedly good for fluid flow in body. What about a red light hat?
I immediately thought of your poor abused brain as I was reading this... and then realized I don't have any excuses like you do for brain malfunction ... except aging and covid.. 😤 ... and here you are doing all this heavy research for us! BPC 157 must be high octane fuel for your brain!
Can't shake this current illness and it's put my brain in neutral. I used to be constantly thinking of something but now ... empty space. Words are missing. It's kinda like I'm 10 yrs older than I should be. Like covid pulled every ailment forward 10yrs for everyone.
I see it in those around me too... all acting like they're exhausted... like they've been without sleep for 48 hrs... just tired out.