First, we need to preface this article with a significant amount of context and clarification, and the first and the most significant annoyance (which guarantees I will delete your comment INSTANTLY).
This does NOT indicate it changes the DNA or even INTEGRATES.
With this out of the way, now to the real context. Back in 2021, a specific paper was published, to a significant amount of criticism from the pro-mRNA part of society, which at the time held all political and societal power and influence. The paper states Pfizer’s mRNA vaccine reprogrammed both the innate and the adaptive immune responses deeply.
Almost 2 years later, they published a revised version of the paper, which was peer-reviewed. This reprogramming paper and the one we are about to cover use different approaches; one uses “a bunch of cells”, and the one we will cover uses only one specific type of cell. The older paper focuses on antibodies, immunological changes to different stimuli, which proteins the cells produce, how they respond, and changes over time.
They are complementary, rather than exclusionary, but the “bird’s eye view” is beyond complex. You can imagine a quantum mirror, looking at different timescales and different “worlds”, with entangled parts.
I will do my best to bridge the highly complex knowledge gap and explain the science here, as I am not aiming at an oversimplified article, many explanations will be sprinkled throughout the entire article.
First, what is epigenetic memory ? Epigenetics can be interpreted as “external influences upon your genes”, meaning it changes the genes your body expresses, which genes are turned on or off without changing the underlying DNA sequence itself. Epigenetic Memory implies a persistent change in said gene expression, the cells and their copies “remember” that change, especially if the change is related to outside stimuli, let us say, an infection. Many other forms of external stimulus enact epigenetic changes.
Persistent epigenetic memory of SARS-CoV-2 mRNA vaccination in monocyte-derived macrophages
One of the biggest selling points of the mRNA vaccines for SARS-CoV-2 was their potent, although short-lived adaptive immune response, these are antibody-based immune responses for the most part, especially regarding SARS-CoV-2. Simplistic explanation, and “for the most part”.
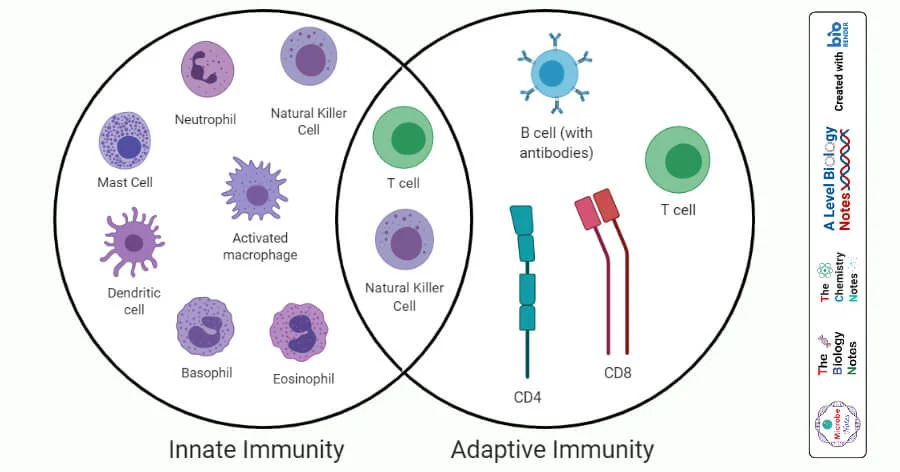
Innate → No memory, fast response, always responds the same, unless “dictated” otherwise.
Adaptive → Memory, remembers the pathogens it fought, enabling more effective responses when it finds that pathogen again
So, how do the mRNA vaccines change how cells without memory respond to specific and non-specific stimuli, over the long-term ? Here the authors decided to test and understand these changes in macrophages, which are one of the key immune cells in the innate arm of the immune system, as they shape subsequent adaptive immune responses.
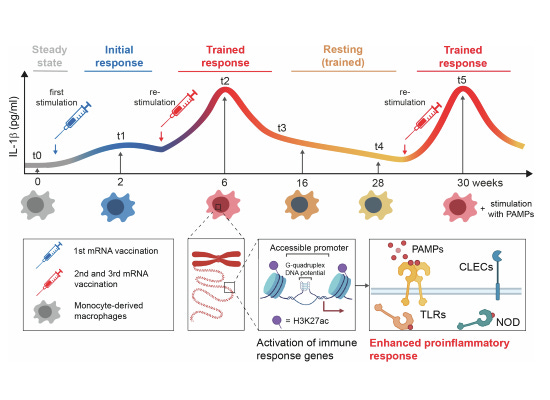
They designed a longitudinal, meaning over time, study. Collecting blood samples before vaccination (t0), 2 weeks after the first dose (t1), 2 weeks after the second dose (t2), and 10 weeks after the second dose (t3). Later, they decided to extend the study, and added time points before and after a third booster dose (t4 and t5, 6 months after the initial two doses).
Other studies have shown that the viral infection itself leads to significant lasting alterations in monocytes and monocyte-derived macrophages that last beyond the average lifespan of these cells, which usually live for days to weeks. By exposing monocyte-derived macrophages to different PAMPs (Pathogen-Associated Molecular Patterns, small parts of pathogens that trigger immune responses) and DAMPs (Danger-Associated Molecular Patterns, small parts of our cells released during damage or stress, activating the immune system), and measuring how they responded and how much of inflammatory protein they released.
What they used to trigger these responses is paramount to understanding this.
ssRNA mimics viral RNA and activates TLR 7 and 8
Zymosan is a fungal wall component activating TLR2 and Dectin-1, which measures responses to fungal infection in a sense
Pam3CSK4 is a bacterial lipopeptide, activates TLR2 and 1
SARS-CoV-2 protein, the specific antigen in the mRNA vaccines
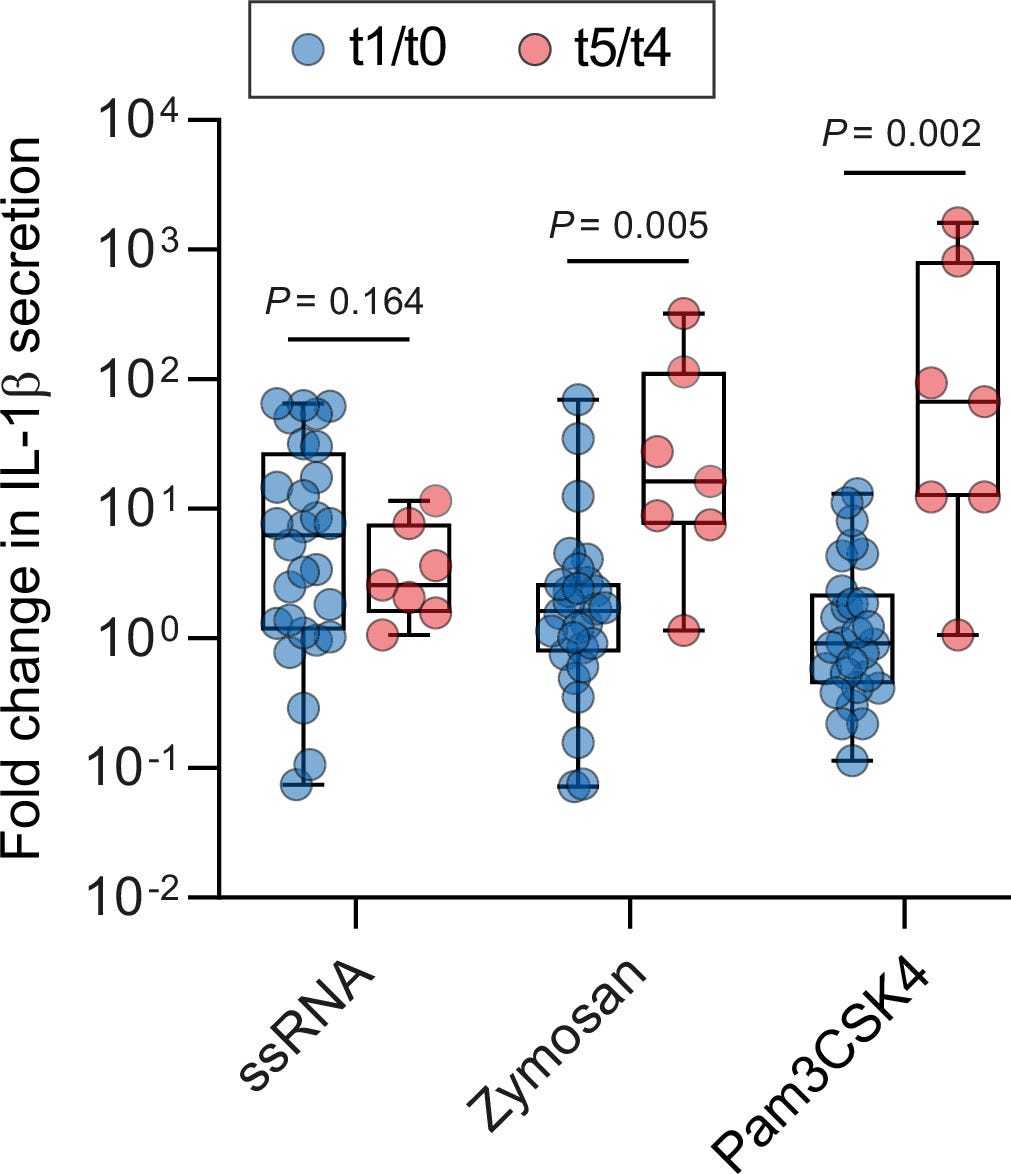
They measured IL-1β release from these stimuli at T0, no vaccine, T1, T2, and also 10 weeks after the second dose, this being T3, and saw a significant increase of IL-1β, but a second dose was necessary for this significantly “stronger” response. For the viral mimic ssRNA, this stronger response was preserved for 10 weeks after the second dose.
IL-1β secretion is associated with the activation of inflammasomes and pyroptosis, a highly pro-inflammatory form of cell death, which is primarily characterized by membrane rupture (this will be incredibly important later), stimulating these macrophages with Spike Protein showed additional pro-inflammatory pathways were elevated.
This means that macrophages from mRNA-vaccinated people respond strongly not just to Spike Protein, but to fungi and bacteria.
Measuring global gene expression changes
As presented in a couple of articles in recent months, whenever you want to have a deeper understanding and a broader perspective on the changes in your line of inquiry, you measure the differences in gene expression, using a form of control. Here the authors performed this on macrophages before the vaccines (T0) and at T2 (thus vaccinated), both stimulated with SP and unstimulated.
Stimulating the macrophages from unvaccinated individuals with Spike Protein only induced a moderate change in gene expression, but in T2 they observed a substantial and drastic change. The genes being upregulated were related to innate immune responses, cytokine signaling, and viral defense. They identified 2519 genes that were differentially expressed, exclusively in vaccinated macrophages, while only 50 were uniquely altered in unvaccinated macrophages.
To understand the next part, just watch this video. I will add anything else in the text
Histone modification in vaccinated macrophages
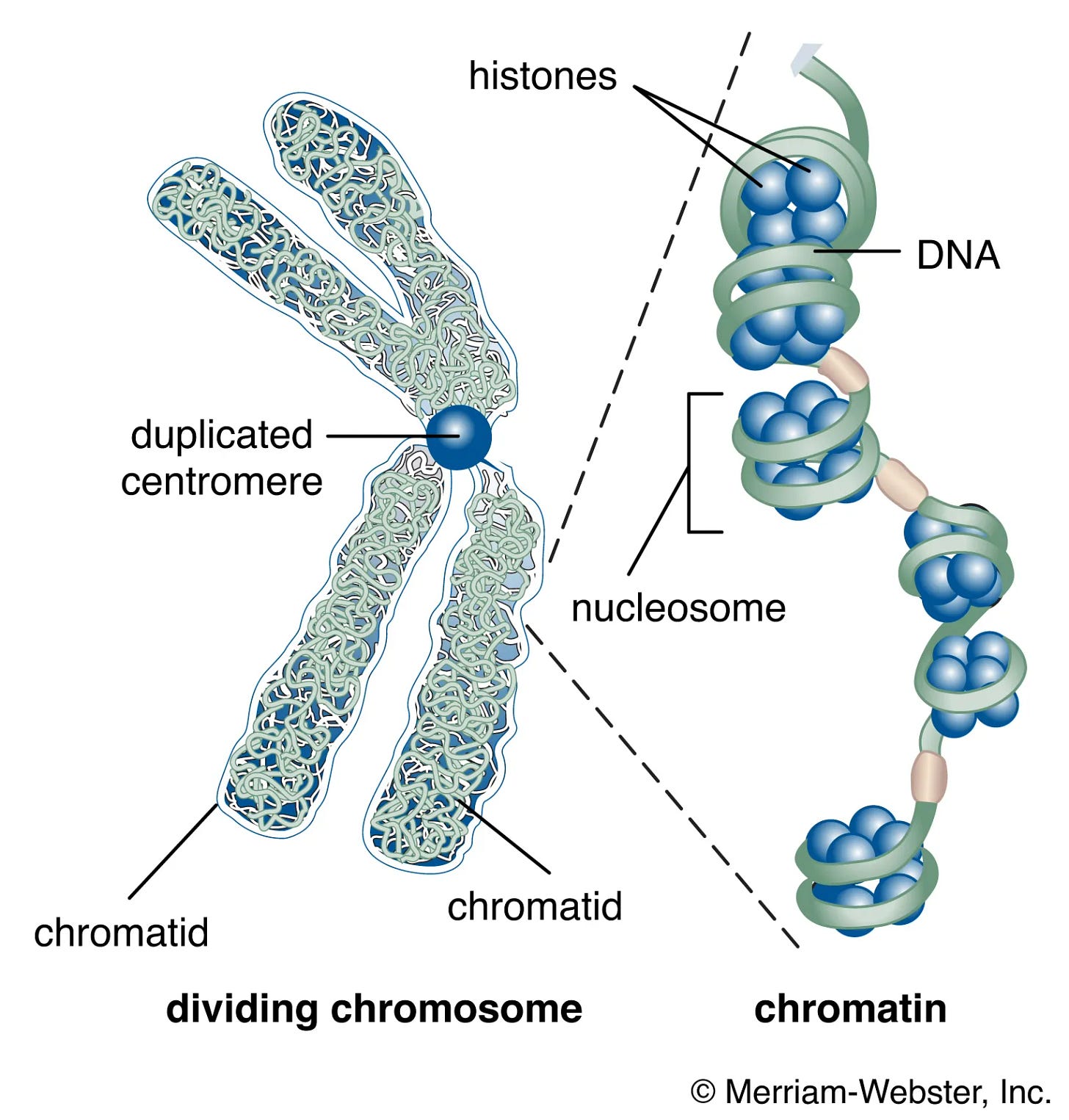
Histones are structural proteins that bind to DNA, wrapping it around a few DNA molecules, forming what is called nucleosomes. This tightly wrapped DNA is called chromatin, and its structure is important for regulating gene expression. Gene expression controls what is produced (mostly, but not only, proteins).
Histones can go through post-translational modification, meaning their structure thus function are changed by the addition or removal of some molecules, after it is produced. There are 4 core histones, H2A, H2B, H3, and H4. A common epigenetic marker, studied in macrophages among many other cells, is called H3K27ac, meaning Histone H3 has a change in Lysine (K) at position 27, by acetylation (adding an acetyl group to a molecule).
This changes the chromatin structure and increases gene expression, thus increasing the production of specific proteins related to the genes here marked by H3K27ac. This effectively means H3K27ac is not just contextually dependent on which cell it is expressed, but it is contextually dependent on even the genes it is expressed. H3K27ac will tag certain genes so the body produces more of the proteins related to those genes.
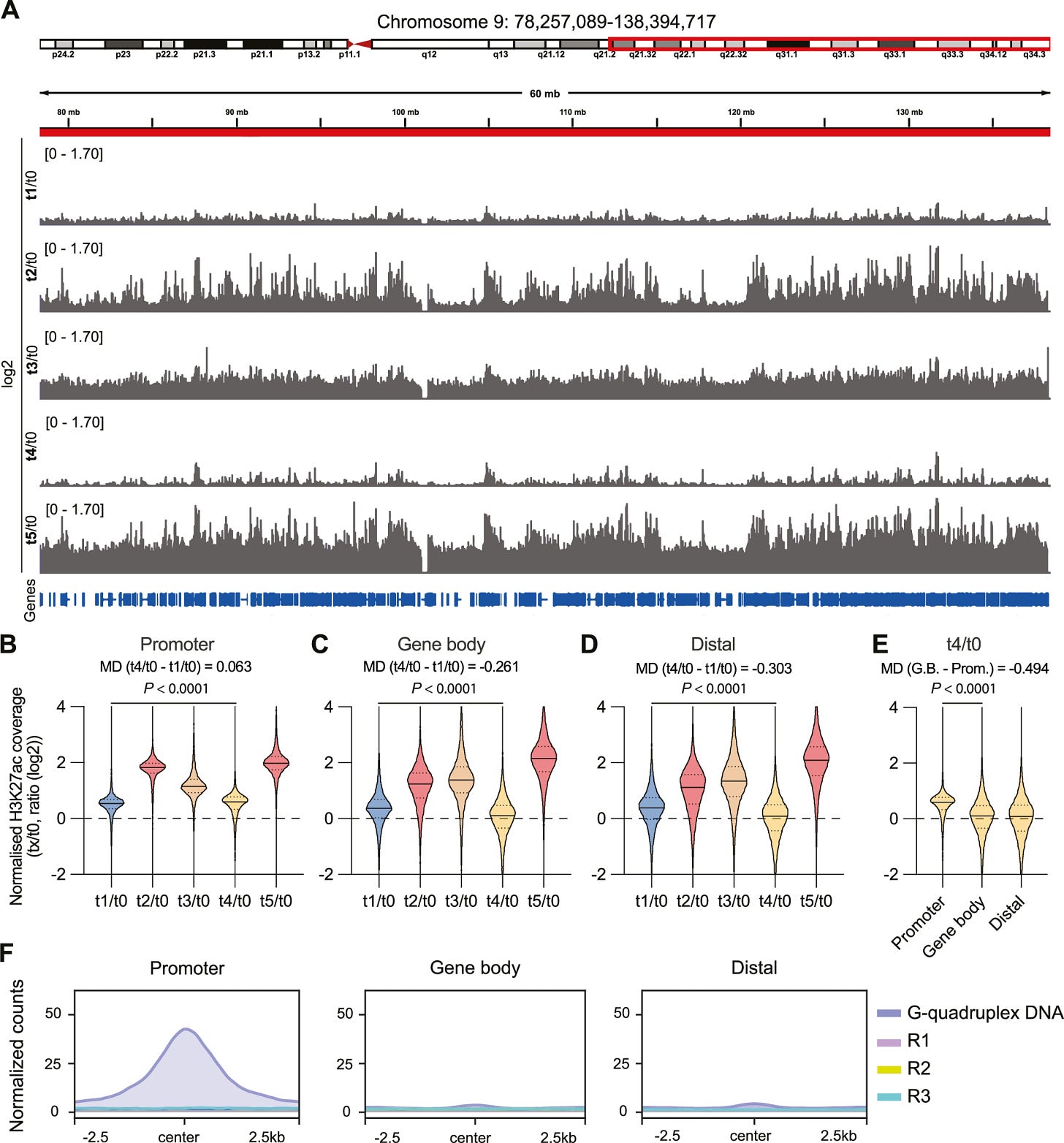
mRNA vaccination induced changes across the genome of the macrophages, but this effect was dose dependent. One dose moderately increased H3K27ac, while a second dose increased substantially. At T3 and 4 (referred to as a resting period), this modified histone went through a slight decrease, and then boosting with another vaccine dose(T5) increased it again.
Here is where the starting findings start to pile up. They found H3K27ac levels at gene promoters persistently elevated at T4, meaning even 34 weeks past the second vaccine dose, this marker was still elevated at gene promoters. H3K27ac is known to change chromatin structure, making it “open”, thus accessible to transcription factors. By being located at promoters persistently, it leads to quicker and higher levels of gene transcription, contrasting with its known “enhancer” function.
If that is the case, a change in how accessible promoters are, and how “easy” it becomes for transcription factors (gene on and off switch) to start the process, the DNA structure could likely be changed. To measure this change in structure and accessibility, the authors decided to measure G-Quadruplex structures (G4).
G4 are secondary structures that form in DNA. Instead of double-helix, G4s are four-stranded structures, a molecular four-story apartment that changes chromatin structure, leaving it more open, as DNA doesn’t wrap as tightly around histones. By measuring G4 sequences along genes close to H3K27ac peaks, they found a substantial increase in G4 DNA-forming sequences in promoters, suggesting H3K27ac persistence over months at promoters is likely due to the structural changes G4 causes in the chromatin of macrophages.
Their next step is discovering exactly which genes H3K27ac is “tagging”, and they found that not all but just certain promoters display a persistent level of H3, both after 2 vaccine doses and at 34 weeks post-second dose. The promoters with H3 were all related to immune responses (positive regulation of leukocyte activation; leukocyte activation involved in immune response; lymphocyte activation and differentiation).
Among the immune-associated genes, the ones of more interest were IL-1β, IL-18, SYK, NOD2, and several C-type lectin (CLEC) family members, which are linked to SYK and inflammasome signaling.
To confirm this persistent immune memory change in macrophages, they measured how much IL-1β was released at T4 and T5 in response to PAMPs. They observed a significant increase in the level of IL-1β of stimulated macrophages at T5 when compared to T4, linking increased cytokine secretion to H3K37ac levels, and this leads to enhanced immune responsiveness.
16 times stronger response against fungal stimulation, and an absurd 67 times against bacterial stimulation. A significantly lower response to viral mimics at T4 and 5 compared to T1 and 0.
In summary, this means the mRNA vaccines induce lasting epigenetic changes, an external change in the DNA structure, by which specific genes are marked by a modification in the protein that shapes DNA, leaving it more open, and marking immune-related and inflammatory genes, changing how vaccinated macrophages respond to many different stimuli over long-term.
The Crux of the Matter
With the paper out of the way, we can explore the things hidden in complexity, and oh there are many. The biggest and most glaring unmentioned effect is the lasting epigenetic changes, which are monocyte-derived macrophages, and the changes observed here imply bone marrow delivery of the mRNA+LNP, which I have covered and referred to recently.
To say H3K27ac is contextually complex is being simplistic. Your first reaction, or that of many “influencers” is likely googling the aforementioned histone modification + cancer, and you get a few dull hits. To understand the context-dependent effects of H3K27ac and cancer, let us use Metformin, one of the very few drugs I suggest some take.
Metformin is now lauded as a longevity, anti-aging drug, but in the past decade, it has been heavily studied for its anti-cancer effects. In oral carcinoma, it inhibits its growth by upregulating total (global) levels of H3K27ac. Here is a review of H3K27ac's role in cancer (sadly, I don’t have access to it).
If H3K27ac is contextual, as in gene-dependent, G4 isn’t that much. G-quadruplexes are enriched in many forms of neurodegeneration, here they form scaffolds (structures) that promote α-synuclein aggregation. G4 plays a complex role in driving neurodegeneration. G4s are implicated in cancer because they halt proper DNA replication, increasing errors. In macrophages, this wouldn’t be a real problem, but in hematopoietic stem cells, mutations would accumulate over time.
If these epigenetic changes are present in all myeloid cells, it increase the risk of chronic stimulation, leading to deplete progenitor pools or reduced plasticity, which should ring a bell for the recent Bone Marrow article, which we see both, and it impairs proper T-Cell and B-Cell regulation, which we have seen trasiently.
While these remain theoretical, the real, observable, and measured problem is the dysregulation, off-target immune response towards different pathogens. The macrophages here, with marked epigenetic changes, respond much more intensely to fungi and bacteria, which implies an overactivation of TLR2 and 4, with 4 being the most pertinent because of the numerous feedback loops it creates.
This TLR overactivation could, in turn, increase the risk of worsened sepsis during secondary infections, a concern turned into a forecast I raised and discussed in the context of altered immune responses post-mRNA vaccination. The genes reprogrammed in the study include CXCL1 (a potent neutrophil chemoattractant, recruiting these cells to sites of inflammation) and CCL20 (a chemokine that attracts Th17 cells).
Chronic IL-1β production, as seen here, upon exposure to different stimuli will overactivate the inflammasome NLRP3, and this pathway and Th17 cells are intrinsically connected to neuroinflammation.
This intense and significant production of IL-1β among other genes will cause a drastic shift in immune responses after each exposure to an antigen or allergen, which in turn will create other feedback loops, as seen in the first reprogramming paper referred at the start of this article.
Remember the membrane rupture-highly inflammatory quote ? There is a specific protein that serves as a “membrane-damage” protein, and it is Galectin-9. Increased levels of IL-1β will upregulate the expression of Galectin-9, forming a paradoxical immune suppressive yet inflammatory loop, which in turn can instruct T-Cells to die (apoptosis).
And there is one protein that plays a role in literally all stages of this. The One Protein To Rule Them All.
HMGB1
There will inevitably be a sequential article to this one. All this is taking into account just the data the paper presents, contamination with plasmids, endotoxin, etc adds a lot more variation and complexity to this.
Consider becoming a paid subscriber, your support is greatly appreciated !








It was suggested a long way back that IL-1-beta (as well as IL-6) were responsible at least in part for the effects of SARS-CoV-2 induced cytokine storm, thus prompting the use of biologics that suppress them (anakinra and tocilizumab respectively). If spike protein "vaccines" are demonstrably showing increased interleukin production then blocking this would be appropriate - but it begs the question of whether one should be using the vaccines at all.
I have previously drawn a parallel with the introduction of TNF-alpha blockade for inflammatory joint disease. There was a theoretical risk of oncogenesis - TNF is Tumor Necrosis Factor. In the UK a register was established with the aim of assessing that risk and the register (BSR Biologics register) has now been going since 2001, with many useful spin-off research publications. As it happens, over this period there has not been any evidence of oncogenesis but without the Register we would probably never have known. I believe the risks of the Covid "vaccines" demand the establishment of a similar long-term surveillance program if they continue to be employed. I am not aware of any attempts anywhere to create a register that would enable this.
The experiments with PAMPs at T4 and T5 are interesting with reduced viral reactivity. As well as permitting more sepsis, the result should be more frequent viral infections, which anecdotally we see in our repeatedly vaxxed acquaintances (and our friends, too!)
This appears to be a positive feedback feature of the SARS-CoV-2 spike protein, enhanced by shedding properties of the injection. It would be interesting to study people from highly mRNA vaxxed populations (Israel or Qatar for instance) versus groups from adenovirus injected populations (China or Russia), looking at macrophages and measuring IL-1Beta.