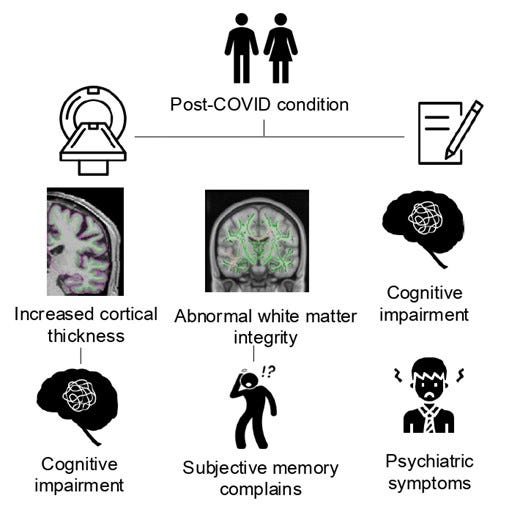
In the article below, the goal was covering recent SARS-CoV-2 papers analyzing the long-term cognitive dysfunction caused by a Covid infection, and some of the overlooked mechanisms contributing to such, and as usual connecting “many dots”. This article builds up on this, with a complete focus on solely the impact, with a little bit of mechanistic explanation, all recent articles participate in the pathways that play a role here.
In the last few months, quite a number of pertinent studies were published that fit almost perfectly into the theme of the last one. They all add to the Cognitive Strike article and expand on the evidence. But before delving into a myriad of different studies, I must refer to my last article. No place Kynurenine is more important than the brain, and its effects are both lasting, complex, and layered but above all else, paradoxical. And Warburgian.
Alzheimer’s Disease and other forms of neurodegeneration in its class have been called “Type 3 Diabetes” for a few years now, because at the core of any issue in any sufficiently complex system is energy inefficiency, spending too much energy to get too little out, and this is even more true in neurodegeneration and many, if not most modern human diseases.
In the research linked above, the authors seek to understand the underpinning of this impaired glucose (sugar) metabolism and attempt to restore it by finding targets. Given the recent progress in the roles of the Kynurenine Pathway and the steps involved in it, they went to test if there is any involved between the KP, its enzymes, and the failure to use glucose properly.
DO1 is expressed in both astrocytes and microglia, essential cells for the nervous system and brain health, critical to the bioenergetic needs of the brain. To understand the roles IDO1 plays here, the authors exposed astrocytes to both amyloid-β42 oligomers (oAβ) and Tau oligomers (oTau), the neurotoxic forms of both proteins, and a combination of both. When exposed to each, there was a prominent IDO1-dependent production of Kynurenine (KYN). If you recall recent articles, KYN is often framed as a last line of defense, but it acts as a double-edged sword. While it may offer some protection, its overproduction disrupts critical metabolic pathways.
Given the axis between IDO1-KYN which binds to AhR, and triggers the location of AhR, they sought to test if this happened, because the protein that regulates this process is called the AhR nuclear translocator (ARNT) testing was done to see if this occurs here. Exposure to Aßo+Tauo activated IDO1 and induced the AhR-ARNT binding.
Why is this important ? Because inside the cell ARNT levels are limited because they can also bind to Hypoxia-Inducible Factor 1-Alpha (HIF-1a), a gene that regulates glycolytic (sugar metabolism) genes and increases the generation of lactate (neurons use lactate for a lot of things). Upon Aßo+Tauo exposure, IDO1 increases significantly, and with its inhibition, HIF-1a increases and reverses the suppression of HIF-1a. This means astrocytic sugar metabolism and bioenergetics are disrupted by the “bad” amyloid and tau and depend on IDO1.
The authors go on to find that IDO1 directly disrupts hippocampal glucose metabolism, especially in amyloid and tau pathology models, and in vivo models too, and IDO1 inhibition rescues the proper metabolism and pathways in the brain. This is one of the most important and overlooked pathways because SARS-CoV-2, and the Spike directly affect this both in short, acute, and long, chronic timeframes, it is one of the most impactful and systemic feedback loops, that only shows its ugly head through years.
Now, to the crux of the matter.
Post-hospitalisation COVID-19 cognitive deficits at one year are global and associated with elevated brain injury markers and grey matter volume reduction
This is a one-year cognitive, serum biomarker, and neuroimaging study at a national level, 351 Covid patients who required hospitalization, with a control group of 2.927, an inquiry on if and how acute infection can cause persistent cognitive deficit, measuring multiple cognitive domains. They were divided into two groups, the Neuro Covid group, which included patients with neurological or psychiatric sequelae, and the Covid group, patients without these symptoms.
They found that patients in both the NeuroCOVID and COVID groups exhibited significant cognitive deficits compared to the normative control group. These deficits were global, affecting all cognitive domains, and were more pronounced in patients who had experienced encephalopathy, cerebrovascular complications, or inflammatory complications. Around 60% of the Neuro Covid group and a little over 40% in the Covid group reported memory problems after infection, with a large number of both with self-perceived progressive memory problems (perception of memory problems is often an indication of actual cognitive deficit, although causes vary vastly).
Markers for brain injury were elevated in both Covid groups compared to healthy control, the average levels of NfL and GFAP (excellent markers to measure continued brain “injury”). were significantly higher in the Covi patients, showing axonal and astrocyte injury. Both markers were even higher in the patients with neurological sequelae, and the authors found a potential link between increased GFAP levels and continuous cognitive impairment, indicating astrocyte injury as a potential axis.
Neuroimaging showed a significant correlation between cognitive deficits and reduced gray matter volume, particularly in the anterior cingulate cortex, responsible for fundamental cognitive processes, including motivation, decision-making, learning, cost-benefit calculation, as well as conflict and error monitoring.
The volume of the anterior cingulate cortex was significantly and moderately positively correlated with overall cognition in both t groups. Additionally, faster response times in memory tasks were correlated with the volume of the parahippocampal gyrus, anterior cingulate cortex, and insula, indicating that structural changes in the brain are a potential cause for the cognitive deficit.
Follow-up over time (longitudinal) of 106 showed a trend toward recovery in cognitive performance, but the improvement plateaued by the second follow-up. Meaning while there is a trend towards recovery, there is also a “cap”, and continuous (chronic) inflammation contributes to the persistent deficit. Ok, the patients here regardless of age all had quite a strong infection (moderate to severe, requiring hospitalization). So what happens in mild infection ?
Changes in memory and cognition during the SARS-CoV-2 human challenge study
Among the many papers covering the neurological impacts of SARS-CoV-2 infection, regardless of variable, this one was the particular one that gave me the “aha moment”, although debate occurred when the paper was published about its methods, (the authors used a novel method), my readers know I don’t rely in one signal but a myriad.
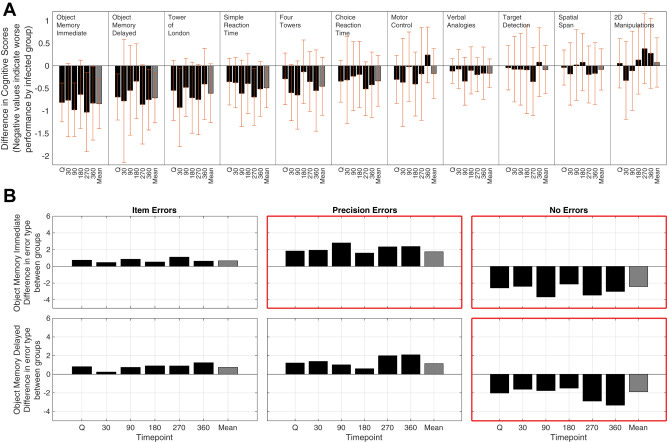
Here they enrolled 34 healthy young volunteers (ages between 18-30), who were never infected to… get infected with SARS-CoV-2 under strict and controlled conditions. The patients underwent daily physiological monitoring and the aforementioned novel, computerized cognitive assessment during quarantine (when they got infected), and follow-ups at 30, 90, 180, 270, and 360 days post-infection. 18 unvaccinated were infected, and the remaining 16 remained uninfected.
Persistent reduction in global cognitive performance was observed in all infected, both observed at first (acute phase) and at the one-year follow-up. Infected individuals scored significantly lower on the bcGCCS (baseline-corrected global cognitive composite score, a cognitive test to calculate overall cognitive function based on scores) compared to uninfected. This persist across all time points, with no significant interaction between group and time, pointing towards a gradual recovery, if there was any in these patients.
The test with the highest sensitivity to measure changes was the memory precision and execution planning test. Object memory task (measures short-term memory recognition) showed the largest effect, η² = 0.233 for Immediate and η² = 0.138 for Delayed. Infected patients exhibited reduced precision in encoding item features, rather than simply forgetting items, showing a deficit in the encoding phase of memory rather than consolidation or retrieval.
Testing for spatial planning and executive function (Tower of London test) had a similar trend, where the difference between groups was significant. This means the viral infection disrupts the prefrontal cortex and processes mediated by it, such as goal-directed behavior and problem-solving (both necessary for complex tasks). The last step was measuring biological markers to observe if there was any correlation.
They found elevated levels of GFAP, the same marker as the previous study, adding another signal to the potential effects of neuroinflammation, especially continued and subclinical (below levels that can be consistently tracked), memory precision, and execution function reduction. Other markers such as Tau and NfL didn’t change significantly.
The viral infection, even mildly will affect the brain, for months, and the trend towards cognitive recovery is slower than the immunological one, “actually” they are the same, since White Blood Cell levels, and even Red Blood Cells play a significant role in the progression of neurodegeneration (lower levels especially). Why was this my aha moment ?
Spatial working memory which was affected long-term here is one of the most essential types of memory, it is crucial for navigation and orientation (allows you to remember routes, and locations, recall where you store something, and spatial relationships), also allows for selective focus choice (critical for driving anything).
But it goes far beyond. Since these changes also affect problem-solving, they affect mathematical tasks, architectural and engineering, and geometric understanding. SWM is critical to breaking down complex problems into smaller, more manageable components, deterioration affects organizing, instructing, and holding multiple components of such tasks. This is a complicated way to say, that these changes affect our Mathematical, abstract learning, it quite literally affects Machine Learning research.
“Worst of all” it also affects Language learning, more on this later. To the next one.
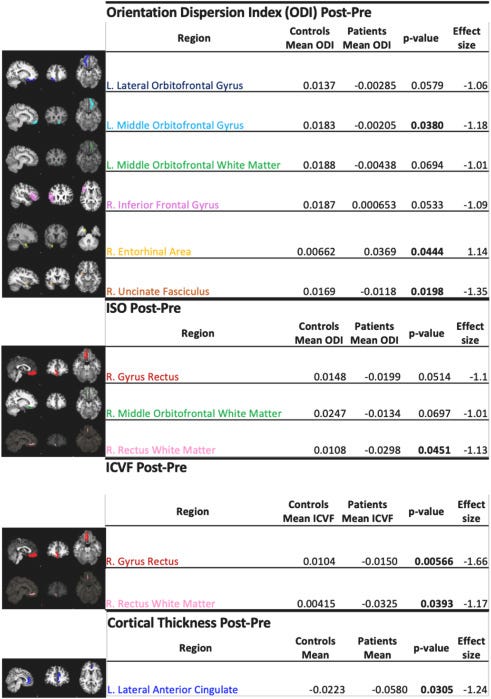
Brain effects of mild COVID-19 in healthy young adults: A pilot study
Results
We identified a decrease of intracellular volume fraction (ICVF), decrease of isotropic volume fraction (ISO) and decrease of orientation dispersion index (ODI) in multiple inferior frontal regions of interest in COVID-19 patients; this longitudinal change was significantly different from the control group which demonstrated increases in equivalent measures. This pattern suggests injury with neuronal loss and/or inflammation as underlying mechanisms. Neurocognitive studies identified a pattern of cognitive decline (processing speed, executive function, verbal learning, working memory) in patients, that did not reach significance.
Conclusion
Our pilot data suggests that mild COVID-19 may result in brain pathology and impact neurocognitive function in younger adults in a manner parallel to prior findings in older individuals. Though findings may not generalize to other SARS-CoV-2 variants, larger longitudinal studies of mild COVID-19 should be undertaken to understand the potential clinical implications of these findings over the longer term.
In contrast to the other papers, this paper followed patients for a short time back in 2020, after a mild infection, when vaccines were not available and yet found a similar trend as observed in older adults. Mild infection induces neurological and structural changes in the brain, affecting neurological function, and cognitive performance, and replicates changes observed in older adults.
Although the authors state “(processing speed, executive function, verbal learning, working memory) did not reach statistical significance” I must inquire if that observation would hold true if they followed the patients for up to a year. So, what about older adults then ?
Tracking cognitive trajectories in older survivors of COVID-19 up to 2.5 years post-infection
Emerging evidence suggests that neurological and other post-acute sequelae of COVID-19 can persist beyond or develop following SARS-CoV-2 infection. However, the long-term trajectories of cognitive change after a COVID-19 infection remain unclear. Here we investigated cognitive changes over a period of 2.5 years among 1,245 individuals aged 60 years or older who survived infection with the original SARS-CoV-2 strain in Wuhan, China, and 358 uninfected spouses. We show that the overall incidence of cognitive impairment among older COVID-19 survivors was 19.1% at 2.5 years after infection and hospitalization, evaluated using the Telephone Interview for Cognitive Status-40. Cognitive decline primarily manifested in individuals with severe COVID-19 during the initial year of infection, after which the rate of decline decelerated. Severe COVID-19, cognitive impairment at 6 months and hypertension were associated with long-term cognitive decline. These findings reveal the long-term cognitive trajectory of the disease and underscore the importance of post-infection cognitive care for COVID-19 survivors.
In this study from China, done in Chinese hospitals, the authors found a trend observed since the early days of the pandemic, especially easier to track with the Wuhan strain. Incidence of cognitive impairment and decline at a rate of19.1% with severe cases representing almost 40% (compared to almost 15% in non-severe and 14.25% in controls.
Patients with severe cases had a higher proportion of suspected dementia and mild cognitive impairment (MCI) than individuals with non-severe cases (dementia: 12.5% versus 1.74%, P < 0.001; MCI: 27.40% versus 13.21%, P < 0.001) and controls (dementia: 12.5% versus 1.68%, P < 0.001; MCI: 27.40% versus 12.57%, P < 0.001). In severe patients, cognitive decline was steep compared to the other groups in the first year, but between 12 to 30 months it accelerated (still present). So far, mild, severe, young, and old. How about what is the poster child research topic for neurological sequelae in regards to SARS-CoV-2 ? Long Covid.
Multimodal neuroimaging in Long-COVID and its correlates with cognition 1.8 years after SARS-CoV-2 infection
We included 53 participants with LC (mean age, 48.23 years; 88.7% females). According to the Frascati criteria, more than half of the participants had deficits in the executive (59%) and attentional (55%) domains, while 40% had impairments in the memory domain. Only one participant (1.89%) showed problems in the visuospatial and visuoconstructive domain. We observed that increased radial diffusivity in different white matter tracts was negatively correlated with the memory domain. Our results showed that higher resting state activity in the fronto-parietal network was associated with lower memory performance. Moreover, we detected increased functional connectivity among the bilateral hippocampus, the right hippocampus and the left amygdala, and the right hippocampus and the left middle temporal gyrus. These connectivity patterns were inversely related to memory and did not survive false discovery rate (FDR) correction.
This study employs imaging techniques to assess structural brain changes and their correlation with cognitive performance loss in patients with Long Covid who reported prolonged neurological symptoms. They used DTI (Diffusion Tensor Imaging) which measures how and in which direction water moves in white matter, the other test was rs-fMRI which measures brain activity during rest.
More than half of the patients showed executive function and attention deficits (59% and 55% respectively), and 40% memory impairment. The DIT test showed a significant correlation between where the water moves in various white matter tracts and impaired memory performance. Higher RD indicated reduced myelination (how the sheath around nerve fibers called axons form), and compromised axon integrity. The regions most affected were the corticospinal tract, cingulum, and superior longitudinal fasciculus.
MRI showed increased resting-state activity in the front-parietal network, especially around the right middle temporal gyrus and posterior cingulate cortices. Higher activity in these areas correlated with loss of memory performance, and the authors argue this is a compensatory mechanism to attempt to mitigate memory impairment. They also found increased functional activity in the hippocampus, amygdala, and middle temporal gyrus in patients with lower memory performance. There is a compensatory response in brain activity because of the structural changes.
Among the many regions affected, which are all important in their own right, the Middle Temporal Gyrus is one of the singular interest. It is a highly significant part of the brain, it is implicated in language processing, semantic memory, social cognition, and visual, auditory, and linguistic cues, and also regulates attention.
Worth noting, especially in regard to our next paper, that the authors see significant changes in the Default Mode Network (DMN), a large-scale brain system that is critical for memory-related, social, and self-referential, introspection, and mind-wandering functions.
Altered functional brain connectivity, efficiency, and information flow associated with brain fog after mild to moderate COVID-19 infection
Given the change in connectivity, it is of interest to understand how these changes occur, how they affect the overall efficiency, and their associations with cognitive deficits. The authors used functional MRI (similar to the previous paper) to map the functional connections network within the brain, which is referred to in science as “connectome”, a comprehensive analysis of our neural pathways.
Using NBS (Network Based Statistic) a form to identify the most important subnetworks within networks of higher complexity, they found a widespread pattern of hypo-connectivity in the SARS-CoV-2 group. This poorer connectivity was spread around multiple areas of the brain, around all cerebral lobes, subcortical structures, and cerebellum.
The DMN was particularly more affected than other regions, and the orbitofrontal regions were also heavily affected, they are important for emotion processing, and decision-making related to the reward system (we are literally driven by reward systems in the brain). This means a mild to moderate infection has a diffuse (widespread, not focused) impact on the brain’s communication network structure, affecting efficient information exchange in many regions.
Next, they tested the level of efficiency in information processing within the brain networks. Local efficiency, a graph theory metric used to quantify these changes was used. Focusing on the regions with hypoconnectivity and related to fatigue, significantly reduced local efficiency was found in the SARS-CoV-2 group, meaning loss of connectivity is correlated with degraded, compromised information processing.
The authors propose that hypoconnectivity in fatigue-related regions, and this efficiency loss may be a compensatory mechanism to reduce the brain’s metabolic demand. Your brain is a glucose beast, it is one of the most energy-intensive organs, and the DMN is one of the most glucose-hungry areas. The brain is attempting to adapt to faulty neurological metabolism, at the cost of efficiency.
To have a deeper understanding of these changes, they measured “effective connectivity” which measures the direction and causal flow of information between brain regions. Using Bayesian network analysis, they constructed directed acyclic graphs (DAGs) representing the information flow in the SARS-CoV-2 and control groups. Strikingly, the DAGs were markedly different, particularly in terms of "false negatives" and "false positives" in the SARS-CoV-2 group.
False negatives, representing connections present in controls but absent in the SARS-CoV-2 group, and false positives, representing novel connections in the SARS-CoV-2 group not seen in controls, were significantly elevated. This demonstrates a fundamental reorganization of information flow pathways in the brain post-infection. Well-established routes of communication are disrupted, and potentially compensatory, but less efficient, pathways emerge and are employed.
A more recent paper on structural brain changes in Long Covid echoes what we have covered here.
Patients with post-COVID performed significantly worse in working and verbal memory, processing speed, verbal fluency and executive functions, compared to healthy controls. Moreover, patients with post-COVID showed increased cortical thickness in the right superior frontal and the right rostral middle frontal gyri that negatively correlated with working memory performance. Diffusion tensor imaging data showed lower fractional anisotropy in patients in the right superior longitudinal fasciculus, the splenium and genu of the corpus callosum, the right uncinate fasciculus and the forceps major, that negatively correlated with subjective memory failures. No differences in blood biomarkers were found. Once patients were classified according to their cognitive status, only those post-COVID clinically cognitively altered presented increased cortical thickness compared to controls.
In conclusion, our study showed that gray and white matter brain changes are relevant in this condition after almost two years of infection and partly explain long term cognitive sequelae.
Before my closing remarks (this article is long and dense enough), one last yee to our cognitive haw. A paper that remained in my open tabs for months, just waiting the right time.
Microstructural brain abnormalities, fatigue, and cognitive dysfunction after mild COVID-19
Although some studies have shown neuroimaging and neuropsychological alterations in post-COVID-19 patients, fewer combined neuroimaging and neuropsychology evaluations of individuals who presented a mild acute infection. Here we investigated cognitive dysfunction and brain changes in a group of mildly infected individuals. We conducted a cross-sectional study of 97 consecutive subjects (median age of 41 years) without current or history of psychiatric symptoms (including anxiety and depression) after a mild infection, with a median of 79 days (and mean of 97 days) after diagnosis of COVID-19. We performed semi-structured interviews, neurological examinations, 3T-MRI scans, and neuropsychological assessments. For MRI analyses, we included a group of non-infected 77 controls. The MRI study included white matter (WM) investigation with diffusion tensor images (DTI) and functional connectivity with resting-state functional MRI (RS-fMRI).
The patients reported memory loss (36%), fatigue (31%) and headache (29%). The quantitative analyses confirmed symptoms of fatigue (83% of participants), excessive somnolence (35%), impaired phonemic verbal fluency (21%), impaired verbal categorical fluency (13%) and impaired logical memory immediate recall (16%). The WM analyses with DTI revealed higher axial diffusivity values in post-infected patients compared to controls. Compared to controls, there were no significant differences in the functional connectivity of the posterior cingulum cortex. There were no significant correlations between neuropsychological scores and neuroimaging features (including DTI and RS-fMRI). Our results suggest persistent cognitive impairment and subtle white matter abnormalities in individuals mildly infected without anxiety or depression symptoms. The longitudinal analyses will clarify whether these alterations are temporary or permanent.
This paper, which is the “oldest” paper among the bunch will remain one of the most impactful articles on the subject of neurological sequelae in regards to SARS-CoV-2 infection, especially mild infection, given enough time. Because medicine is ill-equipped so far to effectively measure, assess, and track microstructural changes, either be it in the brain itself, or the microvasculature.
The paper is straightforward, mild infection already induces neurological changes and cognitive dysfunction, in regions similar to the other papers, with effects similar to the other papers, such as axial damage, and loss of effective connection, especially and once again in the DMN.
There is a significant nuance between each paper, how the data analysis was done, between the groups, testing, and findings, but the larger trend is visible if you use the correct frame. My focus on Language is not merely my interest in Language itself, but because language fluctuation is a very early sign of cognitive decline and a kaleidoscopic collection of neurodegenerative diseases.
An interesting and recent article. How cognition changes before dementia hits: Study finds language-processing difficulties are an indicator — in addition to memory loss — of amnestic mild cognitive impairment. This form of cognitive impairment is called “amnestic mild cognitive impairment” and touches on another one of our subjects. Changes in blood flow are higher in patients who possess this form of cognitive impairment than older adults with normal cognition.
This brings us back to the Kynurenine Pathway, the metabolic shifts, to the Warburgian nature of all of this. If IDO1 is disrupting glucose metabolism in astrocytes, as that first paper suggests, andSARS-CoV-2 is triggering these pathways, even mildly, is it any wonder we're seeing subtle but persistent brain changes, even in those who "recover" quickly from the acute phase?
Fatigue, cognitive friction, and brain fog are likely the symptoms of a brain struggling to function optimally with an altered, less efficient, metabolic landscape. Adaptation through efficiency loss, leading to poorer outcomes in every level of society. The significant, widespread, societal-wide impact of SARS-CoV-2 is impossible to deny, and myopic focus isn’t benefiting anyone, because loud voices have a directional effect on research now.
As a purely for my own entertainment, conspiratorial thought to finish this article. I find it particularly funny, and intriguing that this virus affects specifically the two corner stones of Machine Learning and current Artificial Intelligence research. What are the odds huh…
I will write Part III shortly, covering a couple of new papers and mechanisms aforementioned in this article (different types of blood cells contributing or playing a role in neurodegeneration) and adding more mechanistic explanations. A comprehensive mechanistic analysis can be found by merely reading the last 6 to 8 months worth of articles. To remind you, almost all my Covid articles are a single, highly complex non-linear hypothesis (thesis at this point, but I digress).
Your support is welcome and appreciated, thank you !










My focus has always been SARS and it's leviathanic effects on health and the world, but meaningful research is and will become sparse. While I have the freedom of writing "whatever I feel like" because of my supporters, if you have a topic you would like to write about, comment below.
I have MS and long Covid, had become disabled over five years. I love your articles and have indeed benefited greatly from them. I seem to ve recovering my health finally, both from your suggested supplements and from low-dose naltrexone, anti-inflammatory and anti-aging. Also from homeopathic mercurius soubilis 200 c. I wanted to let you know. Hod bless you!