In case your memory needs a refresher AIDA is an acronym for Alternate Interval Data Analysis, a round-up of news, often or mostly scientific that doesn’t fit a specific theme or is just random, but that I think is worth bringing to attention. And given the underlying theme of most of my recent articles, we start in an uplifting manner.
This is a video of neurons firing in the brain. We each have a mirror image of the entire universe inside our heads.
And thus we start with a rather peculiar article.
Autistic traits foster effective curiosity-driven exploration
Curiosity-driven exploration involves actively engaging with the environment to learn from it. Here, we hypothesize that the cognitive mechanisms underlying exploratory behavior may differ across individuals depending on personal characteristics such as autistic traits. In turn, this variability might influence successful exploration. To investigate this, we collected self- and other-reports of autistic traits from university students, and tested them in an exploration task in which participants could learn the hiding patterns of multiple characters. Participants’ prediction errors and learning progress (i.e., the decrease in prediction error) on the task were tracked with a hierarchical delta-rule model. Crucially, participants could freely decide when to disengage from a character and what to explore next. We examined whether autistic traits modulated the relation of prediction errors and learning progress with exploration. We found that participants with lower scores on other-reports of insistence-on-sameness and general autistic traits were less persistent, primarily relying on learning progress during the initial stages of exploration. Conversely, participants with higher scores were more persistent and relied on learning progress in later phases of exploration, resulting in better performance in the task. This research advances our understanding of the interplay between autistic traits and exploration drives, emphasizing the importance of individual traits in learning processes and highlighting the need for personalized learning approaches.
As a big proponent of not only being a lifelong curious person to anyone but also fostering curiosity in the wider population as a means to drive critical thinking and better cognition, I have an interest in curiosity as a cognitive trait on and in itself. Here the authors found that the ones with higher levels of autistic traits showed more persistence and continued exploring even when faced with difficult tasks, ultimately outperforming those with lower autistic traits.
People with lower levels of autistic traits were less persistent in their exploration and mostly relied on learning progress at the beginning of the task. In contrast, those with higher autistic trait scores showed greater persistence and continued exploring longer, using learning progress information more effectively in the later stages of the task.
In a basic sense, people who score higher in autistic traits are more resilient to repeating patterns and are able to effectively learn, adapt and improve as they go, while those scoring lower (“normal people”) use what they learn at first and can’t withstand the “sameness”, repetition. Different learning approaches to each trait.
The next one is even more interesting and it is coincidentally related to what I called “cognitive subroutines”, which is part of a larger framework I wrote about (undecided if I will ever publish it, I named it Organic Machine Learning).
Young children distinguish the impossible from the merely improbable
From infancy, children show heightened interest in events that are impossible or improbable, relative to likely events. Do young children represent impossible and improbable events as points on a continuum of possibility, or do they instead treat them as categorically distinct? Here, we compared 2- and 3-y-old children’s learning (N = 335) following nearly identical events that were equi-probable, improbable, or impossible. We found that children learned significantly better following impossible than possible events, no matter how unlikely. We conclude that young children distinguish between the impossible and the merely improbable.
When faced with an impossible outcome, children were more likely to revise their understanding of the toy machine’s inner workings. Researchers believe this revision process, triggered by the surprise of the impossible event, led to enhanced learning, as the children sought explanations for the unusual occurrence.
Surprise itself doesn’t guarantee deep learning, a somewhat old proposition of the field, it is the type of surprise that matters. When an event seems implausible within the child’s current understanding, like drawing a color that “shouldn’t” be possible, it forces them to reevaluate their mental models. Trying to make sense of the impossible is what fuels deep learning, as children actively seek explanations to reconcile the impossibility of the event with their knowledge.
It is interesting to mix the two findings into a convergent one, where different neural networks and nerve transmission (autists often have distinct nerve endings and signaling) lead to a distinct learning pattern, driven by curiosity, and I would say curiosity is driven not only by the drive to learn and understand, but to learn and understand what is impossible to you.
And now for the inconvenient truths of the modern world and a subject that not only I covered multiple times, it will be of the highest importance in regards to the infectious oddities happening and to come. To start…
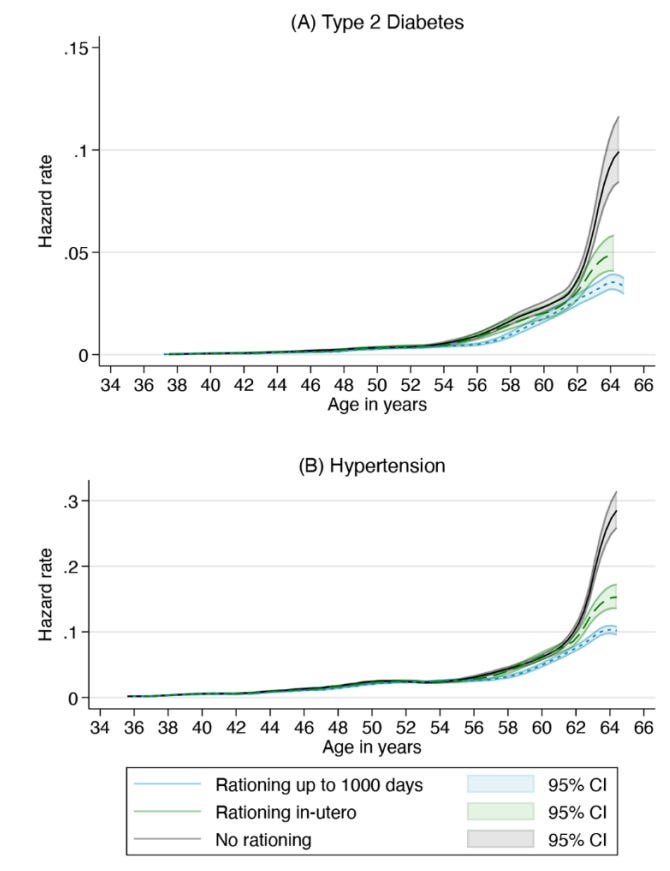
Exposure to sugar rationing in the first 1000 days of life protected against chronic disease
We examined the impact of sugar exposure within 1000 days since conception on diabetes and hypertension, leveraging quasi-experimental variation from the end of the United Kingdom’s sugar rationing in September 1953. Rationing restricted sugar intake to levels within current dietary guidelines, yet consumption nearly doubled immediately post-rationing. Using an event study design with UK Biobank data comparing adults conceived just before or after rationing ended, we found that early-life rationing reduced diabetes and hypertension risk by about 35% and 20%, respectively, and delayed disease onset by 4 and 2 years. Protection was evident with in-utero exposure and increased with postnatal sugar restriction, especially after six months when solid foods likely began. In-utero sugar rationing alone accounted for about one third of the risk reduction.
Everyone knows that added sugar or excessive carbohydrate consumption is bad for you, the argument based on manipulated evidence and bad faith is that “sugar is only bad at period X of your life”, but for the serious person, how soon in life does sugar exposure actually affects you ?
Well, pretty early on. Limiting sugar since conception (meaning months before actual birth) meaningfully reduces the risk for common “sugar-related diseases” in adulthood, such as diabetes, and hypertension. And even for people who later were diagnosed with either of these diseases, the onset was delayed by 4 and 2 years.
The longer the sugar restriction lasted, the lower the disease risk became. In the last 2 decades, a lot of progress has been made in understanding the impact of early life exposure to a myriad of substances, sugar, infections, antibiotics, and drugs, especially the impact of all of these in the microbiome of the mother and the infant. After all “Everything beings in the gut”.
One thing many people, recently (<12 months) infected with SARS-CoV-2, people with poor diabetes, and anyone with metabolic syndrome, insulin resistance, and diabetes go through is “High glycemic variability” meaning glucose levels fluctuate significantly in the blood, and this inevitably affects literally everything inside you. My personal interest is how this affects not only immune cells but immune function.
High glycemic variability is associated with a reduced T cell cytokine response to influenza A virus
Diabetes mellitus significantly increases the risk of severe respiratory virus disease like influenza and COVID-19. Early evidence suggests that this susceptibility to respiratory viral disease is driven by glycemic variability, rather than average blood glucose levels. Here, we use blood samples and constant glucose monitoring (CGM) data obtained from people living with type 1 diabetes (T1D) to determine the effects of glycemic variability on the ex vivo T cell response to influenza virus. We show that high glycemic variability in participants living with T1D is associated with a reduced proportion of CD8+CD107a−IFNγ−MIP1β−TNF+ T cells in response to stimulation with influenza virus and an influenza virus peptide pool. Thus, this study provides evidence that glycemic variability affects the ex vivo T cell response to respiratory viruses. These data suggest that monitoring glycemic variability may have important implications in understanding the antiviral immune response in people with diabetes.
Glycemic variability in diabetes can be assessed using continuous glucose monitoring
High glycemic variability is linked to fewer cytokine-producing T cells to flu•
High glycemic variability is linked to more naive CD8+ T cells and fewer TEMRA CD8+ T cell
A significant variation in glucose (sugar) levels in the blood has been known to affect the immune response to viral infections, especially specific viruses that are glucose-hungry (Influenza, Coronaviruses, some Herpes Viruses, Ebola being among the most common ones), metabolic diseases and poor control of the same directly increase the odds of developing severe disease and dying from said infections.
Here they found that this sugar level variation directly affects the “capacity” of CD8 cells to respond to influenza, and there were fewer “experienced” cells to fight it, thus the body produces more “new” CD8 cells to compensate, and we have another feedback loop. An overlooked impact of excessive sugar and levels of sugar in the blood is its effect on the vascular system. The following article is among the most important ones for the next few years and for overall health in general.
A pathogenic role for IL-10 signalling in capillary stalling and cognitive impairment in type 1 diabetes
Vascular pathology is associated with cognitive impairment in diseases such as type 1 diabetes; however, how capillary flow is affected and the underlying mechanisms remain elusive. Here we show that capillaries in the diabetic mouse brain in both sexes are prone to stalling, with blocks consisting primarily of erythrocytes in branches off ascending venules. Screening for circulating inflammatory cytokines revealed persistently high levels of interleukin-10 (IL-10) in diabetic mice. Contrary to expectation, stimulating IL-10 signalling increased capillary obstruction, whereas inhibiting IL-10 receptors with neutralizing antibodies or endothelial specific knockdown in diabetic mice reversed these impairments. Chronic treatment of diabetic mice with IL-10 receptor neutralizing antibodies improved cerebral blood flow, increased capillary flux and diameter, downregulated haemostasis and cell adhesion-related gene expression, and reversed cognitive deficits. These data suggest that IL-10 signalling has an unexpected pathogenic role in cerebral microcirculatory defects and cognitive impairment associated with type 1 diabetes.
Simplifying part of the author’s opening paragraphs, the brain is a metabolic demanding machine but it lacks energy-storing capabilities, at least at a sufficiently significant level. So it is heavily dependent on an uninterrupted supply of oxygen and nutrients in the vascular networks in the brain, which are finely regulated. Capillaries, part of the microvascular system, very small “veins” represent the vast majority of this system. Disruption of this flow of oxygen and nutrients, especially repeated disruption will contribute to long-term neurological disease, and cause a decrease and significant cognitive degradation over time.
To explore and understand the propensity of capillaries to get stalled (clogged) in the brain is similar to the same effects observed in other organs such as heart, kidneys, liver, and eyes, they used a diabetic mouse model. Using an advanced microscopic test to observe the capillary flow for weeks. The frequency of these stalls was not random but showed a pattern, with specific capillaries stalling multiple times over the observation period.
For example, a capillary that stalled at the four-week mark had a 21–28% chance of restalling at later time points, indicating that certain vessels may be predisposed to stalling under diabetic conditions. Additionally, diabetic mice showed significantly reduced RBC flux in stalled capillaries compared to non-diabetic controls, highlighting how diabetes impairs blood flow efficiency in cerebral microvasculature.
The researchers further analyzed stalled capillaries by classifying them based on the cellular content within the lumen. A striking 93–94% of stalls in both diabetic and non-diabetic mice involved cells, typically RBCs, while a small subset lacked visible cellular components, labeled as ‘only plasma’ stalls. In diabetic mice, there is a mechanistic shift in which certain capillaries experience repeated stalling events, mostly due to factors affecting red blood cell (erythrocyte) dynamics rather than inflammatory cell infiltration.
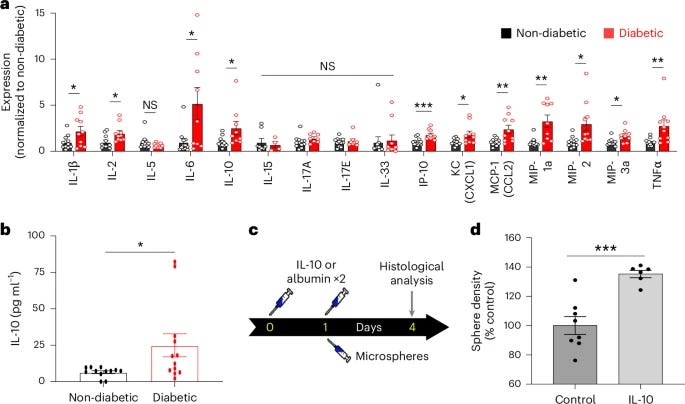
To investigate why capillary stalling was more pronounced in diabetic conditions, researchers conducted a multiplex analysis of cytokines and chemokines in the blood of mice after eight weeks of uncontrolled diabetes. Significantly elevated levels of several cytokines, such as IL-1β, IL-2, IL-6, IL-10, interferon gamma-induced protein (IP-10), tumor necrosis factor-alpha (TNFα), keratinocyte chemoattractant (KC/CXCL1), macrophage inflammatory proteins (MIP-1α, MIP-2, MIP-3), and monocyte chemoattractant protein-1 (MCP-1). Notably, diabetic mice displayed these elevated inflammatory markers well before the eight-week mark when capillary stalling rates became significantly higher.
Based on this early onset of stalling, the team re-examined key cytokines, including IL-1β, IL-6, IL-10, MCP-1, KC, IL-17, and MIG1 (CXCL9) at four weeks. Among these, only IL-10 levels were significantly higher at both four and eight weeks in diabetic mice, suggesting a possible role for IL-10 in the progression of capillary obstructions. Interestingly, cytokines like IL-1β and IL-6, commonly linked to inflammation, were below detection limits at four weeks, underscoring IL-10 potential as a distinct factor in early stalling mechanisms. To validate this further, researchers measured IL-10 levels in another type 1 diabetes model, the non-obese diabetic (NOD) mouse, finding a similar elevation in IL-10 compared to non-diabetic controls.
In conclusion, this research reveals a surprising dual-edge pathogenic role of IL-10 in microvascular dysfunction and cognitive decline associated with T1DM. IL-10 may contribute to the ‘sticky’ endothelium observed in diabetes, which primarily obstructs capillaries with erythrocytes and restricts cerebral blood flow. Blocking IL-10 signaling alleviates these blockages, enhancing cognitive function and reducing the risk of further vascular damage.
Is this important in other settings, besides metabolic diseases and microvascular damage ? Yes, because until very recently there was a mysterious role for IL-10 in severe Covid, and especially poignant in Long Covid and “Long Vaccine”, where some of the injured patients find themselves locked in an eternal state of immune depression + low-grade inflammation, with micro-clotting playing a central role in the long-term (6+ months) dysfunction, and slow recovery.
As an attempt to damper the significant persistent inflammation diabetes and its bystander effects sets off, the body produces IL-10, which in turn will cause damage, a paradoxical double-edged sword. Interestingly enough, many of the cytokines cited in the paragraphs above play a role, direct and indirect in the Kynurenine Pathway, a pathway and its metabolites that are present in higher levels in diabetes.
In the next few days, an inconvenient amyloidal truth =P.
Thank you for your continued support old and new alike.





Specifically on Sharma et al.:
I am quite innocent of mouse research and expected lab values. You note that there is capillary stalling dynamics from RBC factors and separate from inflammatory cells. In clinical work it would be expensive and unusual to be able to study Il-10. Historically an ESR measurement would indicate inflammatory burden as demonstrated by RBC "stickiness." Currently the favoured measure (particularly in the jurisdiction where this research was done) is CRP, or better hsCRP.
To extend the conclusions toward a useful clinical study I would have liked to see hsCRP as well as the detailed glucose and interleukin assays. I would expect the Il-10 measurements to be proportional to CRP, and thus give usefully accessible clinical information.
Following I would have wanted to see the same study run with a bit of Covid Spike protein- any bets on the outcome? The institution is an hour down the road, and I could almost guarantee that no funding for such a study would be available!
There is good research on the effects of DM on endothelial function and the Spike damage seems similar. I had been telling patients (especially diabetics) for at least thirty years that they lived on the lining of their blood vessels; this has been made very clear with the Covid and vaxx damage.
In your first review of the autistic characteristic, I have described the trait as "intellectual curiosity." Because of the structure and politics of ASD (and ADHD) diagnosis the eleven-dimensional Venn diagram makes it difficult to separate useful traits from deleterious conditions. I did not do well with my grade three teacher, if I had been 65 years younger I might have needed to have been drugged into submission!
Thanks, I appreciate your reviews and presentation of these articles which I would never encounter on my own.
Curiosity: that's really interesting! Have always had a dreadful time relating to incurious people. *How could you not want to KNOW??*