Lyme’s Disease is caused by the bacteria Borrelia burgdorferi. I refer to the bacteria as Lyme throughout the article for simplicity.
In my long journey into attempting to uncover why SARS-CoV-2 fragments, be they genetic material (RNA), or its protein fragments, last much longer than, arguably, than any other virus in the body, I have come across a lot of interesting findings, such as Influenza antigens, the immunologically active Lego blocks that help your body fight the infection, remain in specific parts of the body, for weeks, as a way to keep a strong, and protective immune response.
Fragments of any pathogen lasting in the body can be a problem, because many of these fragments are biologically active, while they may not initiate an immune response to help the body (or sometimes harm), they also can induce detrimental changes, cause stress in the cells, your veins, sometimes organs, and above all else, set off an inflammatory chain reaction. Lyme Disease is a complex, extremely difficult condition to treat when patients find themselves in the active infection stage, where the body goes through ups and downs, causing a significant loss of quality of life.
The Lyme acting up (acute-phase of the infection) is already a problem, but this is especially true AFTER treatment, when you target the Lyme pathogen, you kill it, and decline, discomfort and an assortment of worse symptoms set in. Hypotheses on why it happened existed, but now we know how it happens and why.
The peptidoglycan of Borrelia burgdorferi can persist in discrete tissues and cause systemic responses consistent with chronic illness
First, we need some explanation to bridge the scientific jargon to normal language. Peptidoglycan (PG from here on), in a simplistic way, is the bacterial cell wall, the bacteria’s protective skin, consisting of a mixture of small sugar molecules (glycan) and amino acids. As a refresher, the amino acid sequence affects the function of proteins, but the shape of the protein also does. The addition of small sugar molecules to that protein will change its effects.
Unlike many other pathogens, Lyme’s Disease is a perfect example of a stealth pathogen, because it is incapable of producing toxins or virulence factors (pathogens will produce virulence factors to shift the environment towards their favor), and Lyme is the only known bacterium in its family of bacteria that does NOT produce Endotoxin.
Lyme’s has a few unusual characteristics related to the above, such as the amino acids forming its PG, their position and interactions, and the fact that it lacks the pathway required to recycle its PG and fragments, shedding roughly 45% of its entire intracellular PG cell wall outside the cell. Lyme’s PG can be detected in the fluid obtained from the swollen joints of patients with Lyme arthritis, during and often after treatment. This is especially problematic because there will be significant amounts of Lyme’s PG after antibiotic therapy.
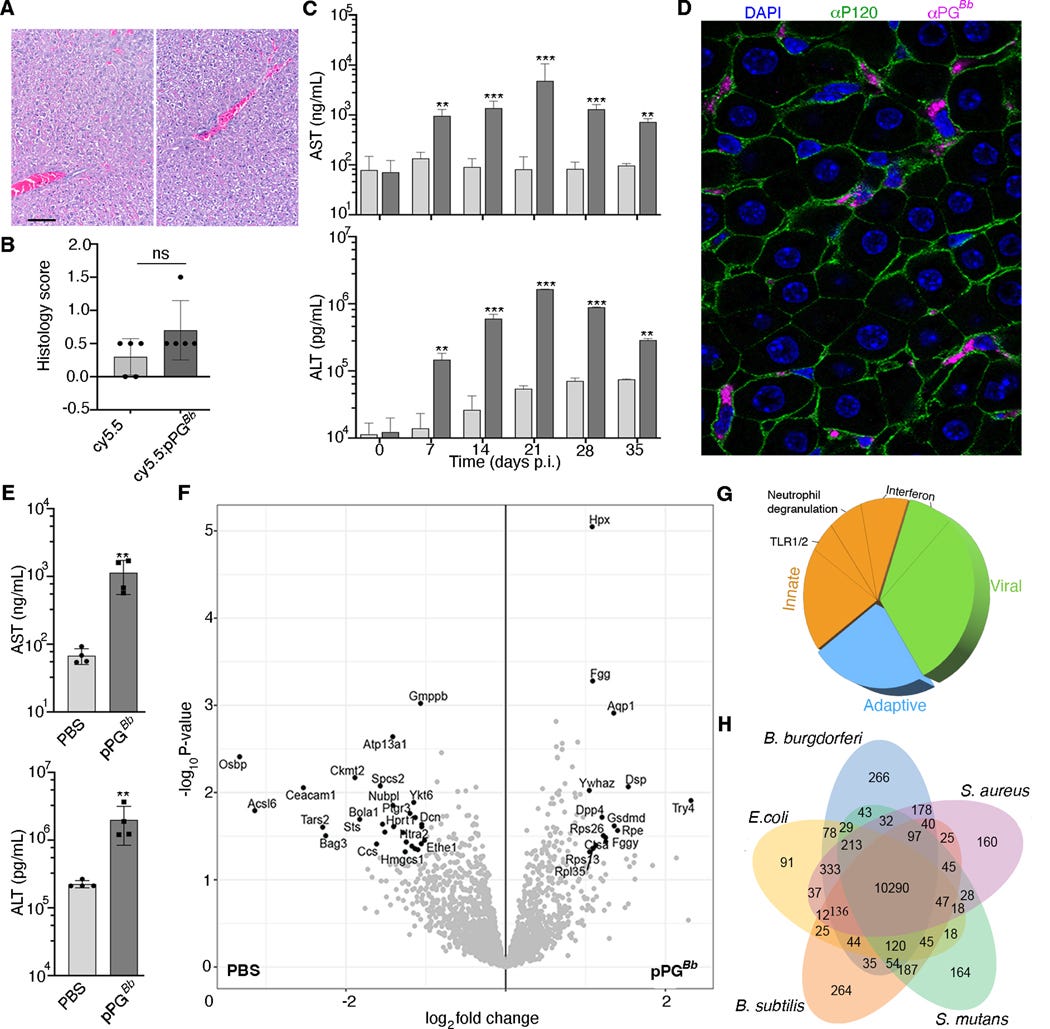
To understand test their hypothesis that Lyme’s PG is both singular and it persists longer than what is believed by current research, the authors found a way to attached a fluorescent dye to highly purified PG, allowing them to inject the glowing PG into living mice and track it for a long period. The control was mice injected with just the glowing dye.
Within two days, the glowing dye was found to be accumulating in the abdomen, and afterwards going towards the liver and spleen, and into the ankle (becoming inflamed). Imaging comparison between glowing PG and unlabeled PG showed that joint, and tendon inflammation are caused by the accumulation of PG, and the liver is the preferred place it accumulates. Even after more than 2 weeks post-injection, the signal of glowing PG was 10 times stronger than just the dye.
As previously mentioned, the active (acute) infection and its treatment both create a significant amount of PG in patients, but now all PG is created equal. So the authors decided to test their hypothesis in a deeper manner. They used “normal” PG, titled digested PG (dPG), which mimics the fragments produced during Lyme’s growth, and polymeric PG (pPG), which are much larger pieces of PG.
Despite injecting the same amount, and both “glowing the same”, dPG was cleared significantly faster, within 2 days, reaching baseline in four days, while polymeric PG persisted, being able to be “seen” even after 4 weeks. They further inquiry if this is feature unique to Lyme or shared by other pathogens, and follow it by using PG peptides from two common bacteria, Escherichia Coli and Staphylococcus Aureus, both with the glowing dye.
Polymeric (meaning bigger chunks here) of E. Coli PG and S. Aureus PG both went to the liver, but their glowingness was 5 to 3 times lower than Lyme’s Polymeric PG. Similar to Lyme’s digest (shorter peptides) PG, E. Coli and S. Aureus digested PG reached baseline within 2 days. Thus, this absurdly long persistence is unique to Lyme’s polymeric PG. This persistence occurs because the polymeric structure of Lyme’s PG allied with its rather singular chemical composition, due to an unusual glycan chemistry at the end of its strands.
If the liver becomes a dumping ground and reservoir for polymeric PG, there must be a mechanism for it. The authors used two specific liver cells, hepatocytes, which are the cells that make the liver work, and Kupffer cells, the macrophages of the liver, that eat all the cellular trash, dead pathogens, etc. Here they used a similar approach using Lyme’s PG, E. Coli, and S. Aureus as controls.
Kupffer cells engulfed (ate) all types of PG, from all pathogens, but they struggled significantly to get rid of Lyme’s polymeric PG. The surprising finding is that hepatocytes, which don’t really phagocyte (eat) that much, showed a distinct preference for “ingesting” Lyme’s polymeric PG.
As polymeric PG is found in the fluids of patients with Lyme complications, especially post-treatment, and the authors uncovered PG concentrates in the liver, so they attempt to measure and observe if there is any effect of said concentration in the tissue. Given that all other forms of PG are rapidly cleared, they focused solely on polymeric PG.
They examined the liver 35 days post-injection and compared it with the control (control here is only dye). Polymeric PG causes minimal visual liver pathology, when an expert analyzes the tissue for damage, but following long-term liver markers such as AST and ALT told a different story.
At the start, they were similar between pPG and control, but at day 7, both proteins were significantly elevated in the mice injected with polymericPG. It peaked at 21 days and declined afterward,s but even 35 days after injection, both markers were still elevated. This shows chronic, low-grade liver inflammation.
Analyzing the overall proteins in the liver (proteomic data) showed a massive shift in the liver. The majority of the altered proteins were related to innate and adaptive immune responses, especially for neutrophils and Toll-like Receptor significant. The astonishing part is two-fold, first, the altered liver proteins shared similarity with viral infections (this is a bacteria), and secondly, when compared with SARS-CoV-2 and Long Covid patients, it was also remarkably similar.
They then tested how healthy human cells from healthy patients respond to polymeric PG, using PBMCs (a mixture of different immune cells, white blood cells, and monocytes) from multiple donors, followed by sequencing the RNA to measure which genes change. Although PGs from other pathogens had shared changes in gene expression, Lyme’s polymeric PG caused the most significant and largest changes.
An emerging pattern among the genes changed by the polymeric PG was genes related to energy metabolism, such as the ones participating in the electron transport chain, and the tricarboxylic acid, arguably one of the most important “bioenergetic pathways” in our cells. Additionally, genes related to CCL19 and IL-23 were significantly increased, and both of these are implicated in post-Lyme treatment symptoms, and they also are heavily implicated in Covid and Long Covid.
They end their research by analyzing the fluid of patients with diagnosed Lyme arthritis, and develop a monoclonal antibody to be able to discern between polymeric PG and digested PG. 27 of 30 patient samples had polymeric PG.
I will make the covering of the next 2 articles shorter, but they are significant.
An Enteric Bacterial Infection Triggers Neuroinflammation and Neurobehavioral Impairment in 3xTg-AD Transgenic Mice
Results
K. pneumoniae, particularly under antibiotic-induced dysbiosis, was able to translocate from the gut to the bloodstream by penetrating the gut epithelial barrier. Subsequently, K. pneumoniae infiltrated the brain by breaching the blood-brain barrier. Significant neuroinflammatory phenotype was observed in mice with K. pneumoniae brain infection. K. pneumoniae-infected mice also exhibited impaired neurobehavioral function and elevated total tau levels in the brain. Metagenomic analyses revealed an inverse correlation of K. pneumoniae with gut biome diversity and commensal bacteria, highlighting how antibiotic-induced dysbiosis triggers an enteroseptic “pathobiome” signature implicated in gut-brain perturbations.
Conclusions
The findings demonstrate how infectious agents following hospital-acquired infections and consequent antibiotic regimen may induce gut dysbiosis and pathobiome and increase the risk of sepsis, thereby increasing the predisposition to neuroinflammatory and neurobehavioral impairments via breaching the gut-blood-brain barrier.
This articles cover a lot of highly interesting immunological changes and systemic effects of K. Pneumoniae infections. For the most part, this is a hospital-acquired bacterial infection and a driver of sepsis, implicated in neurogeneration, neuroinflammation, and the odd acceleration of both after hospitalization. The mechanism remained unknown so far.
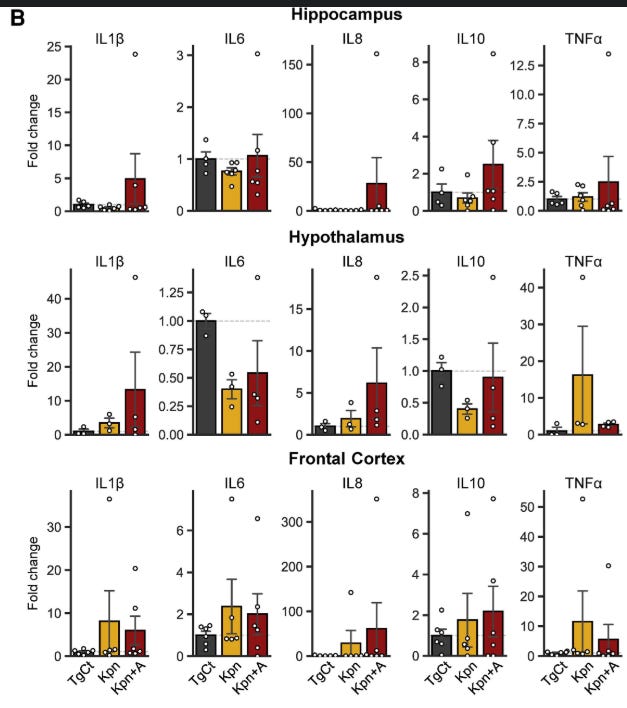
Here, the authors found that K. Pneumoniae, particularly under antibiotic-induced dysbiosis, can translocate from the gut to the blood because the gut barrier becomes compromised by the antibiotic treatments. After entering the bloodstream, it reaches the brain. This can happen without antibiotic treatments, but the treatments make the environment more propitious for the bacteria to do its thing.
Upon the bacteria reaching the brain, measuring inflammatory proteins in different parts of the brain will give you a distinct picture, where proteins that play a significant role in neuroinflammation and inflammation in general experience an increase, and in the antibiotic-treated group, an absurd increase. Given the intricated role of pathogens getting into the brain, inflammation, and the accumulation of misfolded proteins, they measured these in mice brain.
While APP, amyloid precursor protein, suffered a marginal increase, with no difference between the groups in the study, total tau had a significant increase in the antibiotic-treated group. In the author’s own word, “This suggests that K. pneumoniae infection under dysbiosis possibly exacerbates AD pathologies by increasing insoluble tau aggregates.”
The findings in this study are incredibly important not only on their own, but also expanded towards SARS-CoV-2. While co-infections with both pathogens are not common on a global scale, they are indeed common, and SARS-CoV-2 has a drastic impact on how your body responds to bacterial infections, causing dysbiosis and impairing the gut barrier lining on its own. Leading us to the last paper, which deserves an article on its own, but fits the theme.
Viridans Streptococcal Biofilm Evades Immune Detection and Contributes to Inflammation and Rupture of Atherosclerotic Plaques
One lasting orthodox belief in cardiology and angiology is the role of cholesterol in the development of atherosclerosis, otherwise and colloquially known as “clogged artery disease”. But oddly enough, every so often, a paper is published indicating that perhaps bacteria may participate in the process, after all, certain oral bacteria are leading causes of endocarditis. Some would argue that bacteria merely are there, with no role in the process.
This paper focuses on Viridans Streptococci, a group of oral bacteria that are common in your mouth. To test this, the authors used coronary plaques from 121 sudden death victims, and 96 samples from patients with severe atherosclerosis, removed surgically. Upon sequencing the DNA of the samples, the authors found that it contained many species of oral bacteria, but viridans streptococci were the most abundant.
Viridans DNA has been detected in most clot samples from people who had a heart attack, brain bleeds, and clots in other locations. Bacterial DNA was found in over 42% of all samples tested. It was found in 35% of early atherosclerosis, 52.6% in mild to moderate, 42% (as mentioned previously) in partially calcified plaques, and 60% on plaque rupture, thrombosis/hemorrhage. While percentages vary between the severity of atherosclerosis and whether the samples are from post-mortem or living patients, the pattern is evident.
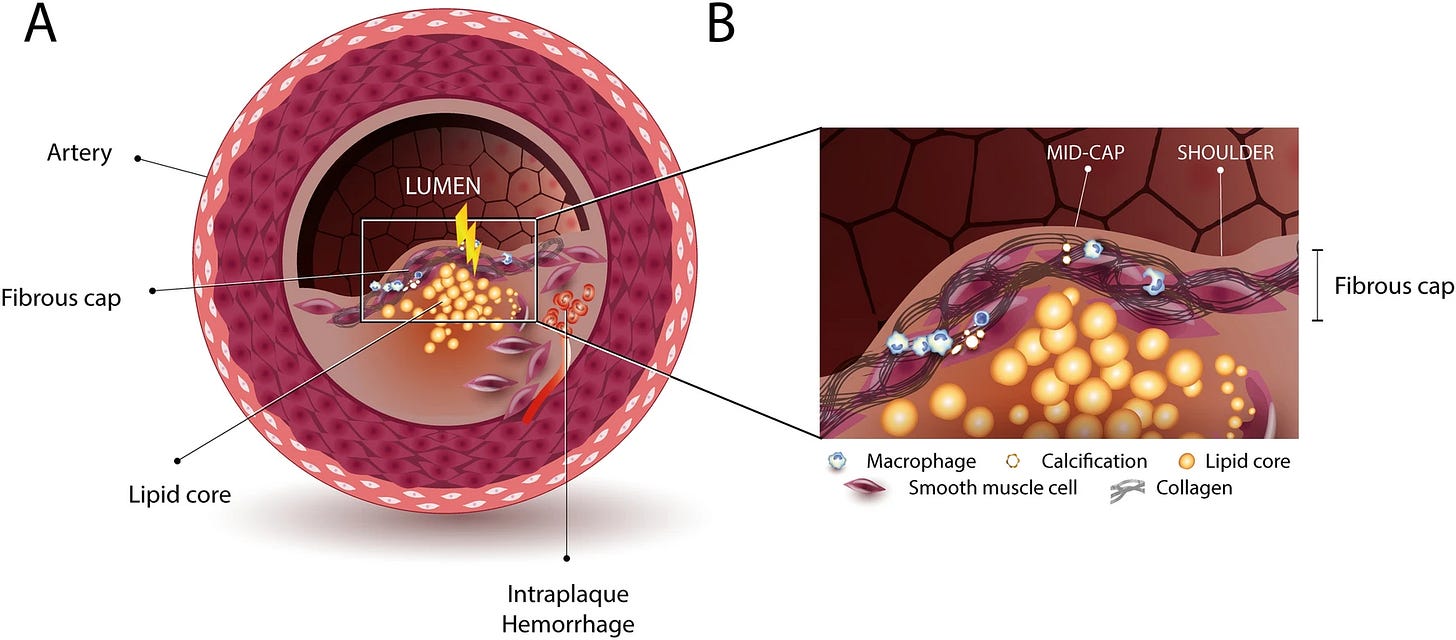
Their groundbreaking finding was done by using antibodies, they were able to visualize and confirm that Viridans formed biofilms inside the coronary plaques after translocating from the mouth, and by doing so, they are able to avoid detection by the immune system, macrophages in this case. Viridans colonize the lipid (fat) core and wall of the plaque itself.
In ruptured arteries or samples from symptomatic patients, the authors were able to observe the bacteria infiltrating the fibrous cap, where they were able to observe both the pathogen, macrophages, and other immune markers. The authors suggest this occurs via the following:
The bacteria may enter the plaque’s interior via leaky new blood vessels, termed neovessels that grow into the plaque. Once inside the plaque core, the bacteria actively switch to a biofilm state, where it is protected from immunosurveillance and immune cells. The prototypical stealth mode, downregulating the molecules that the immune system needs to detect them.
After some initial trigger, such as a viral infection, systemic stress, spesis, or other inflammatory signals, it activates the bacteria inside the biofilm, resulting in an active infection. These are aggressive bacteria, and they infiltrate the fibrous cap, the structural barrier that keeps plaque from rupturing. Given that they are full-fledged pathogens and are inducing inflammation, the immune system is now able to detect them and start an immune response.
This potent immune response autoamplifies itself, and in this regard, among the many proteins and enzymes, it produces enzymes that degrade specific structural proteins, and some of those, such as collagen, are what it give the fibrous cap its structural strength. This makes that specific portion of the blood vessel unable to withstand the stress of blood flow, so it either tears open or completely ruptures. The clot will at some point block the blood flow to the heart muscle, causing a heart attack.
As I have proposed that misfolded proteins are likely a forgotten, dual-edge, lost last line of defense against bacteria, then, to be proved accurate in my observation by cutting-edge research, I also believe that plaques are also a form of non-canonical, long-term defense mechanism, also forgotten by the body.
While biofilms enable bacterial communities to remain in a stealthy state, avoiding immune detection, they are not inert, biofilms are biologically active and can do a list of things, and among these, they can send constant inflammatory signals. Misfolded proteins around bacteria, granuloma around tuberculosis in the lungs, abscess around stubborn infections, it is not unheard of.
Cholesterol itself has some antimicrobial activities so my hypothetical mechanism would after the failure to mount an initial immune response, the body allows cholesterol and its byproducts to accumulate at the bacterial site, macrophages are then recruited to the site, and eat the cholesterol, becoming foam cells (these are fat-laden macrophages, and one of the main mechanisms for atherosclerosis).
Macrophages can’t digest biofilms, and thus, they become part of the complex structure. The body, as a final act, signals smooth muscle cells to secrete collagen and other proteins, forming the thick, fibrous cap over the entire structure, thus forming a wall-like structure around the biofilm, the plaque. This, of course, explains just some plaques, not the formation of all.
And yes, you can easily trace parallels, connections, shared pathways and direct implications between the 3 papers, and SARS-CoV-2.
If you decide to support my work, it is greatly appreciated, thank you !




Even after a briefer analysis of the 2 other (not Lyme) papers, I found it to be already lengthy and becoming complex so I decided to avoid making it overly complex, but there is a significant overlap with many effects of Covid, with all 3 papers, from both an immunological perspective, to a direct physiological damaging one.
As I wrote on my Twitter, Kofi donations (buying me a coffee) go towards buying a new computer. Any support is appreciated, don't feel obligated to do so. Everything remains the same.
I may write a very short post on AI tomorrow, although it covers bacteria, I didn't want to mix it with this one.
More on US Bioweapon Lyme and link to Pfizer Jabs.
https://geoffpain.substack.com/p/us-bioweapons-lyme-disease-and-pfizer