Covid infection causes long-lasting microbiome instability
How the immunological shift might have started
This is the “other half” of the following substack. They are independent of each other, but reading both will give you a broader understanding of the changes going on, if you didn’t read it, I would advise you to.
Before we delve into this paper, and further down this nest, I would like to point out that for the longest part I wrote that Omicron was shifting both the immune system and the microbiome, but nobody was testing (microbiome stuff is rather… “niche” for now, but demand is rising and becoming more popular), so finally we get evidence of the exact shifts going on.
Mild SARS-CoV-2 infection results in long-lasting microbiota instability
The abstract is pretty straightforward and somewhat easy to understand. In this research, they followed 14 people who tested positive for SARS-CoV-2 and remained outside the hospital (meaning mild disease, not requiring any form of hospital visit), and 4 households (families) as control.
The microbiome of the ones who tested positive was unstable for up to 154 days, followed by testing in mice with all variants so far, and all variants affected the gut, a fact known to any competent clinician since mid-2020, now with evidence that even mild infection causes a shift in your gut microbes composition.
While Omicron doesn’t cause severe disease, for the most part, it did in fact impact the gut microbiome and significantly affect Akkermansia muciniphila, even in the absence of organ damage (lung in this particular case), the gut microbiome was impacted. Now we have evidence for a shift in the gut microbiome in all stages of the disease, in which in my opinion the effects are compounding, the further the disease progress, the more severe it affects the microbiome, causing a shift from good to bad, but the Akkermansia is already a very good hint of the “starting point” of this particular cascade.
Long-lasting microbiota instability following SARS-CoV-2 infection.
Next, we sought to identify which bacterial species were most variable following SARS-CoV-2 infection, and visualized the most variable ASVs. Low abundance organisms were among the most variable species as determined by CV. Variable bacteria spanned distantly related phyla, and included Rothia species which have been associated with SARS-CoV-2 and correlated with differences in the built environment.
Complementary analyses of our metagenomic data confirmed these trends. Gene family abundance exhibited significantly more variability (β-dispersion metric) in Cases relative to Controls. Gene families trended towards more variation between sample points for Cases compared to Controls . Gene family variability assessed by CV was also significantly higher in Cases relative to Controls.
Taken together, these data indicate that the human gut microbiome can be destabilized months after initial infection with SARS-CoV-2. However, it is not possible to infer a causal role of SARS-CoV-2 infection in destabilizing the gut microbiota based only on observational studies in humans given the clear potential for confounding factors. By focusing on outpatient sampling, we were able to rule out the confounding effects of hospitalization and treatment in prior studies; however, our longitudinal samples corresponded to a period of extensive social distancing that could have feasibly impacted the gut microbiota. Thus, we turned to an established mouse model of SARS-CoV-2 infection, wherein environmental and genetic variables could be controlled to test the causal role of viral infection in shaping the mouse gut microbiota
SARS-CoV-2 alters the gut microbiota in susceptible mice.
All SARS-CoV-2 variants led to a dramatic and significant impact on the mouse gut microbiota. Visualization of all three variants compared to the Uninfected mice showed clearly distinct patterns of grouping. However, they did not reach statistical significance by PERMANOVA testing after adjusting for longitudinal sampling. Bacterial diversity increased over time following infection with all three variants.
We observed differences at the phylum level for four phyla between groups (two-way ANOVA for Verrucomicrobiota, p=6.09e-8; Firmicutes, p=1.29e-5; Bacteroidota, p=9.08e-4; and Proteobacteria, p=1.10e-2). There was a striking loss of Verrucomicrobiota in Omicron-infected mice and a subtle reduction in Proteobacteria in WA1-infected mice, with reciprocal shifts between Firmicutes and Bacteroidota in these groups.
Notably, Akkermansia diminished over time in response to infection with WA1, Delta, and Omicron.
Interestingly each variant affected different microbes in the gut, therefore it is possible each difference between initial symptom presentation between variants, at least in some cases, was because of this initial impact in the gut, most of the changes in the cases here (all mild) recovered their microbiome equilibrium after a while.
Among the other microbes affected in the gut, Akkermansia was the most affected over time, declining with the passing weeks with both the Wuhan original, Delta, and intriguingly Omicron. This is important because recent research has demonstrated that Akkermansia one of the gut most abundant resident microbes is extremely important for your overall health and it, quite literally, affects your immune system/response.
To me, this is the first time a group of researchers demonstrate that mild infections do bear consequences to overall gut (therefore systemic) health, especially over time, and show the type of changes, unique to each variant, but now dominated by Omicron-based variants.
I will now attempt to demonstrate why and how important this finding is, because this is happening in everyone, and of course, a subset of the global population is much, much more susceptible to the bad effects of such change than the other part of the population.
Akkermansia Muciniphila Protects Against Atherosclerosis by Preventing Metabolic Endotoxemia-Induced Inflammation in Apoe−/− Mice
Altered composition of the gut microbiota is involved in both the onset and progression of obesity and diabetes mellitus. However, the link between gut microbiota and obesity-related cardiovascular complications has not been explored. The present study was designed to investigate the role of Akkermansia muciniphila, a mucin-degrading bacterium with beneficial effects on metabolism, in the pathogenesis of atherosclerosis in apolipoprotein E–deficient (Apoe−/−) mice.
Beneficial Effects of Newly Isolated Akkermansia muciniphila Strains from the Human Gut on Obesity and Metabolic Dysregulation
These are just a couple of papers on the effects of supplementing with hAkkermansia M. , there is extensive research on the effects of bacterial translocation (bacteria leaving certain areas of the body and going where they shouldn’t be) and causing a myriad of detrimental effects, often in regards to the metabolism, referred to in the literature as metabolic endotoxemia. Akkermansia is proposed to help with exactly this scenario and has potential relevance to HIV-related inflammation. Good article on that here.
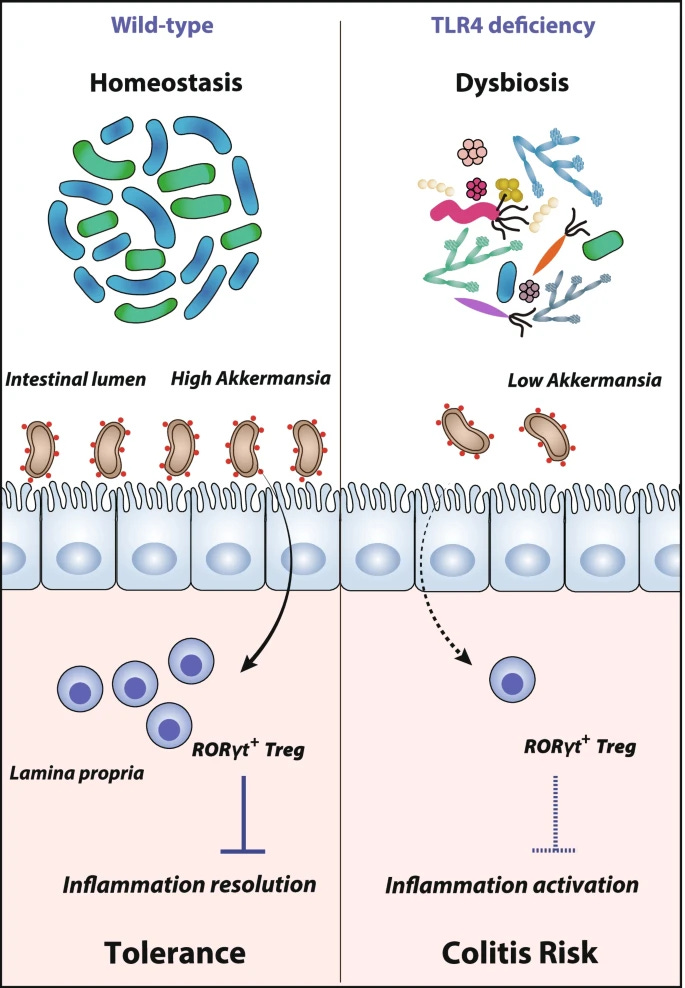
The role of Akkermansia M. goes beyond merely modulating the host metabolism, it can effectively talk directly to your immune system and control your susceptibility to colon inflammation for example, by controlling the expression of T Reg cells, cells that regulate inflammatory responses.
There is a very complex relationship between Akkermansia bacteria and your immune cells, controlling how naive CD4+ cells become T Reg cells and maintaining a balance of your immune system to avoid autoimmunity. This play a very important role in the whole Spike-centric vaccines, because either by vaccination or by (multiple infections) it is clear that a lot of people are losing their ability to keep self/non-self at bay, losing their balance, and developing different types of autoimmunity.
Many latent or chronic infections are known to use a similar strategy to drive both long-term infections (persistence) and linkage to autoimmunity, balance, therefore, is key.
Here the authors found an important link between the presence of Akkermansia at sufficient levels and its capacity to control the negative effects of Interferon Gamma and how it negatively affects glucose tolerance. Not only that, Akkermansia by itself is linked to IFN-gamma gene expression, so if there is a shift in the abundance or lack of this microbe, Interferon Gamma will come to create one of its many loops and do you remember what IFN-G is known to regulate, besides a myriad of other cytokines and immune processes? IDO, is directly related to the Kynurenine Pathway.
(Worth to note that Akkermansia itself has a protective effect against immune-mediated liver injury, basically directly regulating the precise cytokines SARS-CoV-2 is known to activate and induce damage)
From the paper cited above.
Live and pasteurized A. muciniphila, both supplements could ameliorate the inflammatory response and down-regulate IDO1 expression, thereby restoring tryptophan metabolic imbalance. In conclusion, the current study demonstrated for the first time that KQ may act on the A. muciniphila abundance, regulate IDO1 activity, and thus ameliorate ALI. Interestingly, A. muciniphila supplementation could be a promising therapeutic option for lung diseases through the gut-lung axis.
Researchers also found that Akkermansia can regulate tryptophan metabolism in colitis too. Any supplement that influences the levels of Akkermansia in the gut will have beneficial effects on glucose metabolism, and as such in many of these immune-related pathways, such as Bofutsushosan, a Japanese herbal medicine. Pasteurized Akkermansia can ameliorate LPS-Induced Intestinal Barrier Dysfunction by modulating AMPK and NF-kB, the second being one of the most powerful complexes of proteins in the body, and used by both LPS and SARS-CoV-2 to induce pathology.
Intestinal barrier dysfunction is how a person can end up with bacteria in places it shouldn’t be (bacterial translocation), the persistence of different symptoms, and links to the development of autoimmunity. Given the nature of the spike protein, what we know so far, the substack linked at the start of this one, and everything I wrote, it is evident how impactful, long-term, multiple infections per year will affect one’s health.
UDCA (ursodeoxycholic acid) has a very positive effect on the accumulation of Akkermansia and the downregulation of inflammation, as I wrote in the previous microbiome-related substack, and would cite this exact paper again. This is a cheap, generic, off-patent, safe drug, so besides modulating in a positive way the microbiome, it will help the liver and inflammation, I firmly believe some people will benefit from a short-term (4 weeks) use of this drug.
This one is long and rather complex as it is, so I will leave the other papers I wanted to cover (they are not microbiome related directly, but are Long Covid related), but will end this substack with this one. Hints of how the current immunological shift started.
Over 2000 years ago, with just his observational and deductive skills, no internet, no nothing, a single man saw the truth.
More detailed research on how SARS-CoV-2 and its Spike Protein affect the gut and the microbiome is much needed. At least in parts, significant pieces of this puzzle lie there, as one can clearly see by now.
I welcome and appreciate the support of those who choose a paid subscription, or who decide to buy me a coffee whenever they feel like it, and everyone who shares my Substack. Without all of you, this wouldn’t be possible.











Thanks John Paul. Is tudca as a supplement the same as udca?
And my wife is having a helluva time losing weight after having a bad case of covid early in the year. This looks promising on that front to rejumpstart her mitochondria/microbiome.
Thank you so much!
With your special research interests in the gut, this is quintessentially GUT WRENCHING material.
I just sent your Substack to a couple of East Coasters who are suffering now with COVID!